AAV neutralizing antibody detection service
Background Introduction
AAV has become one of the virus vectors with great market prospects in scientific research and gene therapy due to its high tissue specificity, good spreadability, low immunogenicity, high safety, and stability. There are various types of AAV capsid proteins, among which type 2 and type 9 AAV have been extensively studied as viral vectors in gene therapy and have been applied in clinical practice. At present, Luxturna based on AAV2 vector (developed by Spark Therapeutics for the treatment of Leber congenital melanoma type 2) and Zolgensma based on AAV9 vector (developed by Novartis for the treatment of spinal muscular atrophy) have been approved for use in patients and have shown good therapeutic effects.
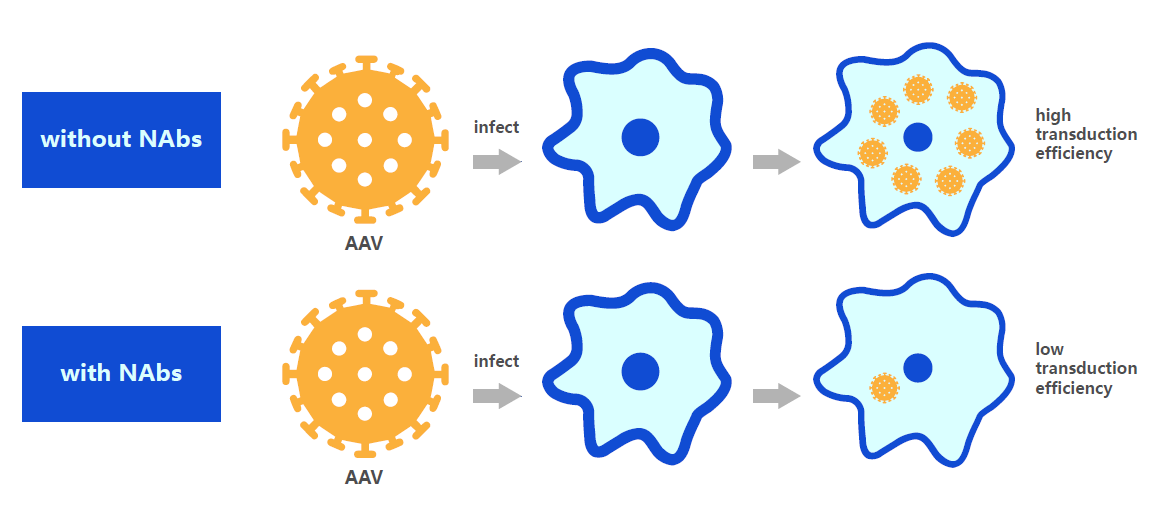
However, neutralizing antibodies against AAV capsids are commonly present in most experimental animals and populations, and can block AAV transduction when the vector enters the bloodstream. Especially when AAV is applied in gene therapy, the pre-existing neutralizing antibodies against AAV in the body can reduce the efficiency of gene transduction, which greatly limits the application of AAV. Therefore, in order to improve the effectiveness of AAV therapy, it is necessary to detect the presence of AAV neutralizing antibodies in subjects or animals in advance, which has guiding significance for the applicability of AAV serotypes in clinical or preclinical trials.
Pre stored neutralizing antibodies greatly reduce gene transduction efficiency
Test method
Weizhen Biotechnology uses a luciferase reporter system to detect neutralizing antibodies against AAV in vitro, which can detect the presence of specific serotypes of neutralizing antibodies in the test sample, facilitating customers to choose different animal models or AAV serotypes. By detecting the Luciferase luminescence value RLU, the presence of neutralizing antibodies against AAV virus in the test sample can be calculated based on the luminescence value to determine whether the serum/antibody has neutralizing activity against this serotype of AAV virus.
If a large amount of neutralizing antibodies against AAV are pre stored in the test sample, it indicates that a higher dose of AAV is needed to achieve better gene transduction effects. Therefore, this serotype is not an ideal choice for the animal body, and AAV serotypes or animal models need to be replaced for further research or clinical trials.
Neutralization antibody testing service project
1、 Service Projects
AAV neutralizing antibody detection service
2、 Service type
Qualitative testing, quantitative testing
3、 Sample to be tested
① Serum samples, dry ice transportation;
② Volume: ≥ 200 μ L;
③ Requirements: sterile, hepatitis B, hepatitis C HIV、 Syphilis, EB, CMV test negative;
4、 Service Process
Determine the testing plan → Sign the contract → Send the sample to be tested → Test for neutralizing antibodies → Issue the testing report
5、 Service cycle
Within 5 working days from receiving the sample to be tested
6、 Service Description
① Can provide neutralizing antibody detection for AAV1/2/5/6/7/8/9, etc;
② The testing data and reports are only responsible for the test samples;
7、 Data support
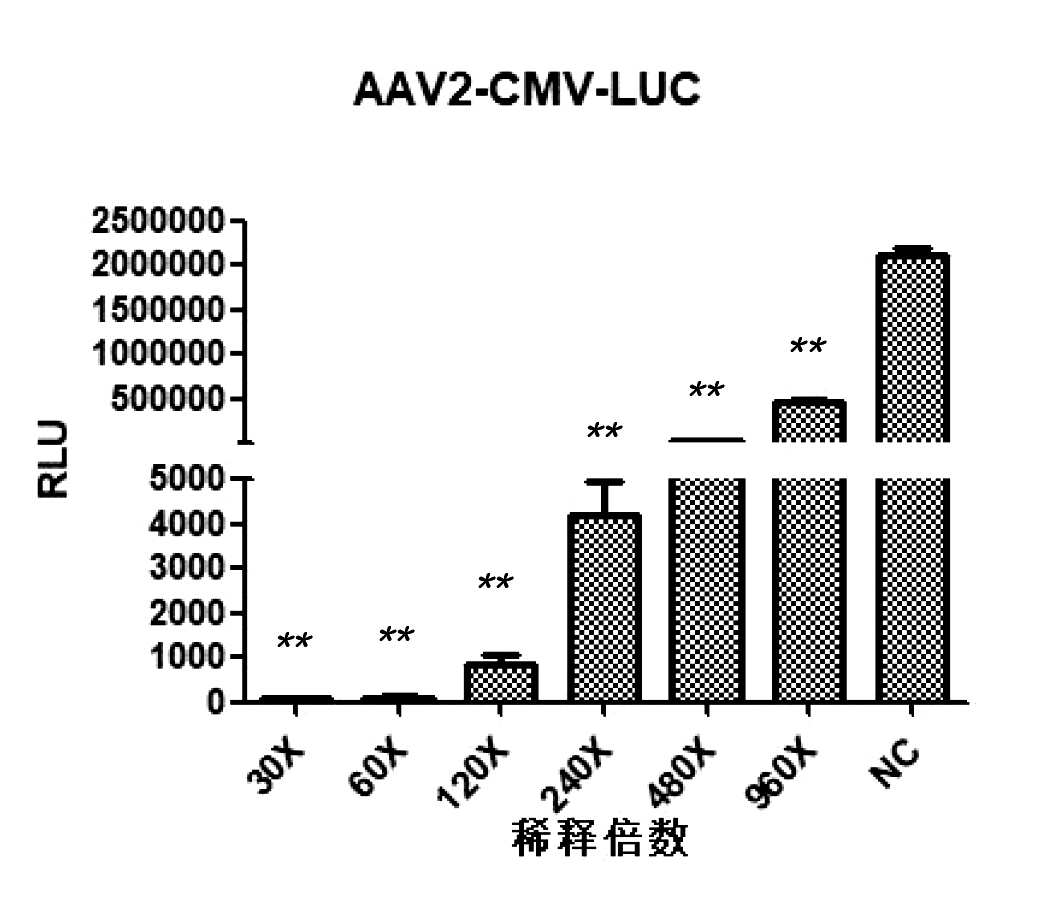
AAV2-CMV-LUC neutralizing antibody detection results
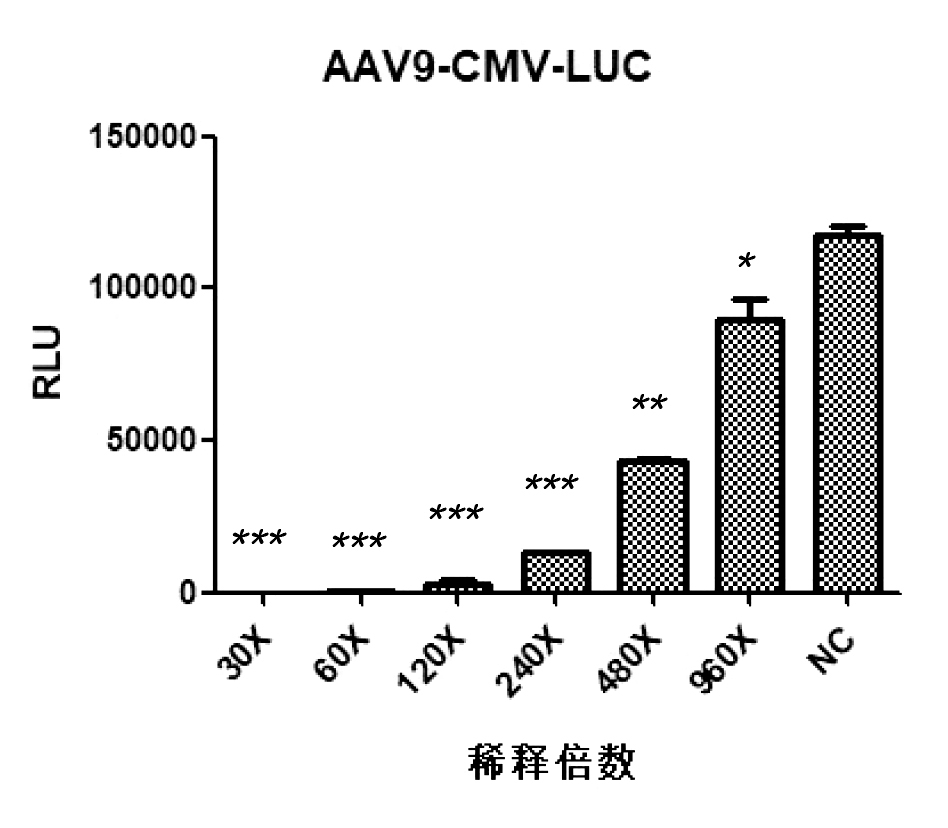
AAV9-CMV-LUC neutralizing antibody detection results
Testing steps:
Dilute the test sample according to the following gradient: 30X, 60X, 120X, 240X 480X, 960X, take 10uL of each gradient and co incubate with 10uL of AAV virus of different serotypes. After incubation, infect 293T cells with the mixture, and detect the RLU luminescence value using an enzyme-linked immunosorbent assay (ELISA) reader 24 hours after infection. Overall, at a serum dilution of 1:60, both AAV2 and AAV9 inhibition rates can approach 100%.
8、 Service advantages
① High sensitivity: neutralizing antibodies can still be detected even after serum dilution of 240-480 times;
② Strong specificity: In serum without antibodies, neutralizing antibodies cannot be detected at all;
③ Good repeatability: The data obtained from multiple experiments have minimal differences.