AAV Vector with modified capsid
1. Research background
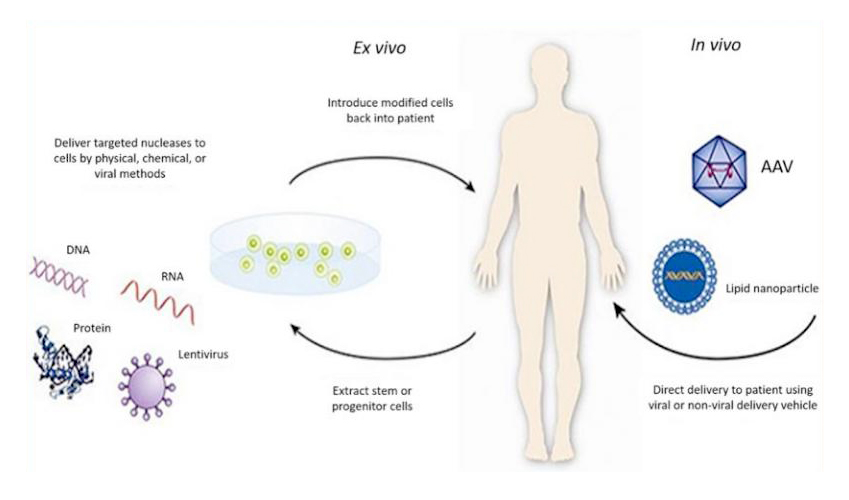
Gene therapy is a new therapeutic method, which can treat a variety of diseases, including cancer, genetic diseases, neurological diseases, infectious diseases, cardiovascular diseases and autoimmune diseases. It is aimed at the root of the disease rather than just the symptoms of the disease. What is the uniqueness of such an effective treatment method? It's actually quite simple. It involves introducing exogenous normal genes into target cells using certain techniques or vectors to correct or compensate for diseases caused by defects and abnormal genes, in order to achieve the goal of treating diseases (Figure 1).
Figure 1: Schematic diagram of gene therapy(from https://www.fda.gov/)
The key to achieving success in gene therapy is the selection of the vector. Among numerous vector tools, Adeno Associated Virus Vector (AAV Vector) has become a highly promising viral vector for gene therapy due to its high safety, low immunogenicity, and long expression duration. As a result, it is increasingly being used in clinical trials to treat various diseases. As of now, three gene drugs based on AAV vectors have been approved for marketing (Figure 2): the first one is GLYBERA, which was approved by the European Medicines Agency (EMA) in 2012 ® (alipogene tiparvovec, AT), This drug is developed by UniQure in the Netherlands and is used to treat lipoprotein lipase deficiency (LPLD); The second one is LUXTRUNA, which was approved by the US Food and Drug Administration (FDA) in 2017 ® (voretigene neparvovec-rzyl, VN), The following year, it was approved by EMA again, and the drug was developed by Spark Therapeutics in the United States for the treatment of Leber's congenital black mask type II; The third one is ZOLGENSMA, which was also approved by the FDA in 2019 ® (onasemnogene abeparvovec-xioi, OA) , This drug is developed by AveXis in the United States and is used to treat spinal muscular atrophy type I in children under 2 years old.
The key to achieving success in gene therapy is the selection of the vector. Among numerous vector tools, Adeno Associated Virus Vector (AAV Vector) has become a highly promising viral vector for gene therapy due to its high safety, low immunogenicity, and long expression duration. As a result, it is increasingly being used in clinical trials to treat various diseases. As of now, three gene drugs based on AAV vectors have been approved for marketing (Figure 2): the first one is GLYBERA, which was approved by the European Medicines Agency (EMA) in 2012 ® (alipogene tiparvovec, AT), This drug is developed by UniQure in the Netherlands and is used to treat lipoprotein lipase deficiency (LPLD); The second one is LUXTRUNA, which was approved by the US Food and Drug Administration (FDA) in 2017 ® (voretigene neparvovec-rzyl, VN), The following year, it was approved by EMA again, and the drug was developed by Spark Therapeutics in the United States for the treatment of Leber's congenital black mask type II; The third one is ZOLGENSMA, which was also approved by the FDA in 2019 ® (onasemnogene abeparvovec-xioi, OA) , This drug is developed by AveXis in the United States and is used to treat spinal muscular atrophy type I in children under 2 years old.
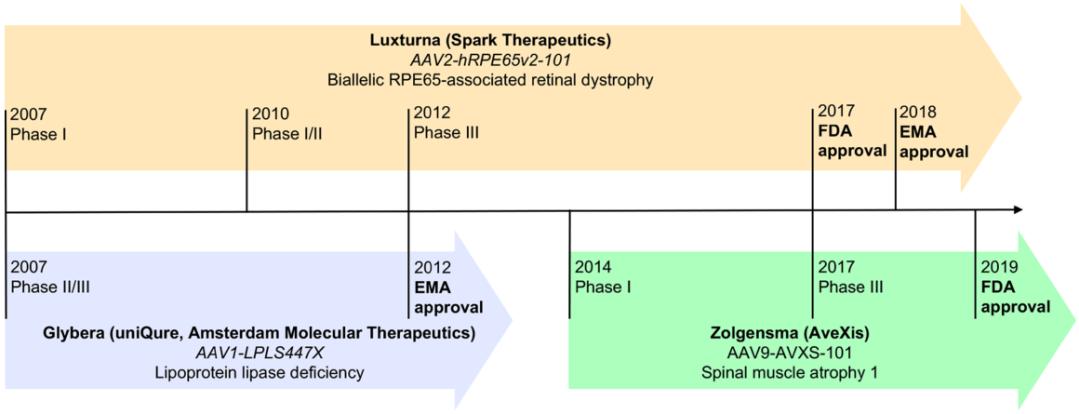
Figure 2: Timeline of Three Approved AAV Vector based Gene Drugs
(Lugin, M. L., et al. (2020). ACS Nano 14(11): 14262-14283.)
The above achievements, coupled with a series of preclinical and clinical studies in the US Clinical Trials Database (ClinicalTrials. gov), have confirmed the safety and efficacy of AAV vectors. However, wild-type AAV did not evolve to deliver disease treatment genes. Moreover, the pre-existing neutralizing antibodies and the carrying capacity of AAV vectors in the body further limit the widespread application of AAV vectors. At present, the focus of scientists' research is mainly on engineering AAV vectors to improve their safety, targeting, and transduction efficiency. There are various methods for constructing engineered AAV vectors, and in this article, we will focus on sharing with you the capsid modified engineered AAV vector (Figure 3).
Figure 3: Schematic diagram of engineered AAV vector
2. AAV genome structure
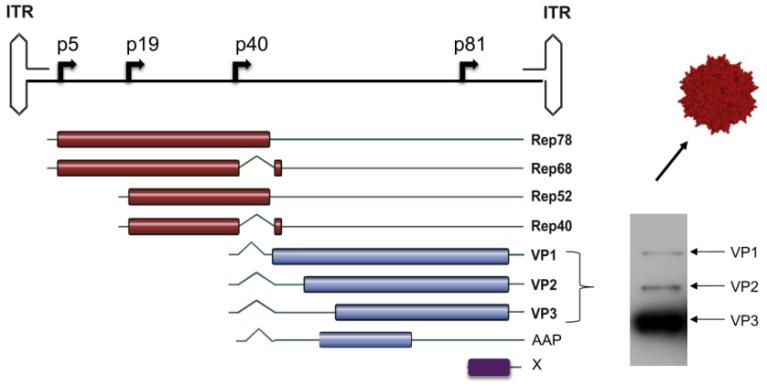
Understanding the genome structure of AAV is the foundation for constructing engineered AAV. The wild-type AAV contains a single stranded linear DNA genome of approximately 4.7 kb, with three genes Rep, Both ends of Cap and X are composed of 145bp ITR, forming a T-shaped hairpin structure (Figure 4). The Rep gene encodes four essential regulatory proteins for viral transcriptional regulation, replication, and packaging: Rep78, Rep68, Rep52, and Rep40. The Cap gene encodes three overlapping structural proteins (VP1, VP2, VP3, with an expression ratio of 1:1:10) and the assembly activating protein AAP (assembly activating protein) necessary for VPs to enter the nucleus. The X gene encodes proteins with supporting functions in genome replication.
Figure 4: Genome structure of AAV2 vector
(Buning, H. and A. Srivastava (2019). Mol Ther Methods Clin Dev 12: 248-265.)
For the Cap gene, we can see from the distribution of VPs genes in the genome that VP3 is fully included in VP2 and VP1. Therefore, VP3 is the common region of the three VPs, also known as the VP3 common region; VP2 has a sequence of approximately 57 amino acids longer than VP3, and this sequence is included in VP1, hence it is also known as the VP1/VP2 common region; Only the N-terminus of VP1 has a unique sequence of approximately 138 amino acids, hence it is also known as the VP1 Unique Region (VP1u). VP3 public area is responsible for assembling icosahedral clothing shells; VP1u contains the phospholipase A2 domain, and the VP1u and VP1/VP2 common regions contain nuclear localization sequences NLSs. Therefore, the VP1u and VP1/VP2 common regions are responsible for endosome transport&escape, nuclear localization, and genome release.
3. AAV capsid structure
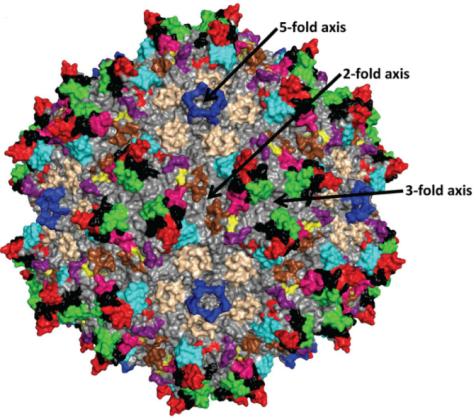
The analysis of AAV capsid structure helps to understand how AAV infects cells and determine the location of engineered AAV capsid modifications. The capsid of adeno-associated viruses is a T=1 icosahedron (an icosahedron is a convex polyhedron surrounded by 20 triangles, with each 5 triangles enclosing a quintuple vertex. There is a quintuple rotation axis through each pair of opposing quintuple vertices, a triple rotation axis through the center of each pair of opposing triangles, and a double rotation axis through the midpoint of each pair of opposing edges). It is assembled from 60 VP monomers through the interaction of these rotating axes, and these VP monomers can be all VP3 or composed of VP1, VP2, and VP3 together.
The prominent features on the surface of AAV capsid are two-fold depressions, three fold protrusions, and five fold channels (Figure 5). The cylindrical channel connects the inside and outside of the capsid and is the place where AAV DNA enters the capsid. It participates in multiple processes such as Rep protein binding, capsid protein assembly, VP1 N-terminal exposure, AAV virus infection, etc; The double concave area is the thinnest part of the virus capsid; The main function of the triple protrusion is to recognize receptors.
Figure 5: Topological structure of AAV2 capsid assembled from 60 VP3
(Tseng, Y. S. and M. Agbandje-McKenna (2014). Front Immunol 5: 9.)
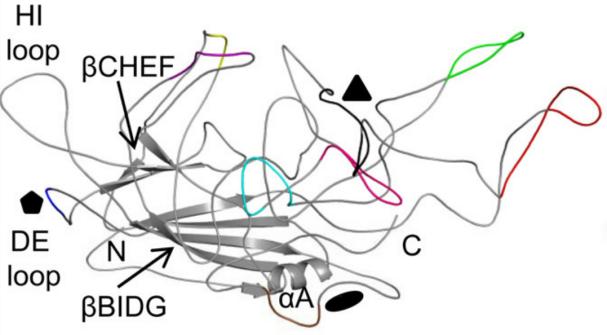
The topological structure of each capsid protein consists of a conserved alpha helix (α A) and a beta barrel core composed of 8 antiparallel chains (β B - β I) (Figure 6). There are four large interchain loops (BC, DE, GH, HI, named according to their positions) between the eight antiparallel chains, which form the outer surface of the capsid. Among them, the GH ring is a larger ring and can be divided into multiple sub rings. These sub rings have high sequence and structural variability in the main capsid component VP3, and have been identified to contain 9 variable regions (VRs). These VRs differ between different sera and mainly play a role in receptor attachment, tissue transduction, and antigenicity.
Figure 6: Topological structure of AAV2 VP3 monomer. 9 VRs are marked with different colors:
I,Purple; II, Blue; III, Yellow; IV, red V, Black; VI, Pink; VII, Blue color; VIII, Green; IX, choco
(Gurda, B. L., et al. (2013). J Virol 87(16): 9111-9124.)
4. Engineering AAV with capsid modification
Specific VP subunits have been used as targets for capsid modification, such as removing immunogenic motifs, integrating tags or fluorescent groups, and retargeting.
AAV2 is a relatively mature serotype for research. Using AAV2 as the backbone, extensive research has shown that I-587 (VP1 amino acid count) and I-588 are commonly used capsid modification sites in the common VP3 region, as these two sites are located near the triple bulge and can accept peptide chain insertions up to 34 amino acids in length without affecting capslization and genome packaging. Moreover, the insertion of exogenous peptide chains modifies the first receptor binding motif of AAV2, ultimately endowing AAV2 variants with new targeting properties. Buning et al. summarized AAV2 variants with peptide chains inserted at I-587 and I-588 (Table 1). Weizhen Biotechnology currently possesses nearly 70 types of AAV Cap plasmids listed in Table 1, which can be directly packaged with AAV viruses.
|
Serotype
|
Position
|
Name
|
Target Cell Type
|
Insert
|
|
AAV2
|
I-587
|
AAV-I-587
|
β1-integrin positive tumor cells
|
QAGTFALRGDNPQG
|
|
AAV2
|
I-587
|
AAV-588NGR
|
CD13-positive tumor cells
|
NGRAHA
|
|
AAV2
|
I-587
|
AAV-MO7A
|
tumor cells
|
RGDAVGV
|
|
AAV2
|
I-587
|
AAV-MO7T
|
tumor cells
|
RGDTPTS
|
|
AAV2
|
I-587
|
AAV-MecA
|
tumor cells
|
GENQARS
|
|
AAV2
|
I-587
|
AAV-MecB
|
tumor cells
|
RSNAVVP
|
|
AAV2
|
I-587
|
rRGD587
|
αv integrin positive tumor cells
|
CDCRGDCFC
|
|
AAV2
|
I-587
|
AAV-C4
|
tumor cells
|
PRGTNGP
|
|
AAV2
|
I-587
|
AAV-D10
|
tumor cells
|
SRGATTT
|
|
AAV2
|
I-587
|
AAV-SIG
|
endothelial cells
|
SIGYPLP
|
|
AAV2
|
I-587
|
AAV-MTP
|
endothelial cells
|
MTPFPTSNEANL
|
|
AAV2
|
I-587
|
AAV-QPE
|
endothelial cells
|
QPEHSST
|
|
AAV2
|
I-587
|
AAV-VNT
|
endothelial cells
|
VNTANST
|
|
AAV2
|
I-587
|
AAV-CNH
|
endothelial cells
|
CNHRYMQMC
|
|
AAV2
|
I-587
|
AAV-CAP
|
endothelial cells
|
CAPGPSKSG
|
|
AAV2
|
I-587
|
AAV-EYH
|
smooth muscle cells
|
EYHHYNK
|
|
AAV2
|
I-587
|
AAV587MTP
|
skeleton muscle cells
|
ASSLNIA
|
|
AAV2
|
I-587
|
AAV-r3.45
|
neuronal stem cells
|
TQVGQKT
|
|
AAV2
|
I-587
|
AAV2-LSS
|
CNS
|
LPSSLQK
|
|
AAV2
|
I-587
|
AAV2-PPS
|
CNS
|
DSPAHPS
|
|
AAV2
|
I-587
|
AAV2-TLH
|
CNS
|
GWTLHNK
|
|
AAV2
|
I-587
|
AAV2-GMN
|
CNS
|
GMNAFRA
|
|
AAV2
|
I-587
|
AAV-Kera1
|
keratinocytes
|
RGDTATL
|
|
AAV2
|
I-587
|
AAV-Kera2
|
keratinocytes
|
PRGDLAP
|
|
AAV2
|
I-587
|
AAV-Kera3
|
keratinocytes
|
RGDQQSL
|
|
AAV2
|
I-587
|
AAV-588Myc
|
none
|
EQLSISEEDL
|
|
AAV2
|
I-587
|
AAV2.N587_R588insBAP
|
adaptor
|
GLNDIFEAQKIEWHE
|
|
AAV2
|
I-587
|
AAV2Ald13
|
adaptor
|
LCTPSRAALLTGR
|
|
AAV2
|
I-587
|
DMD4
|
vaccine
|
QVSHWVSGLAEGSFG
|
|
AAV2
|
I-587
|
DMD6
|
vaccine
|
LSHTSGRVEGSVSLL
|
|
AAV2
|
I-588
|
A588-RGD4C
|
av integrin-positive tumor cells
|
CDCRGDCFC
|
|
AAV2
|
I-588
|
A588-RGD4CGLS
|
av-integrin positive tumor cells
|
CDCRGDCFC
|
|
AAV2
|
I-588
|
AAV-VTAGRAP
|
tumor cells
|
VTAGRAP
|
|
AAV2
|
I-588
|
AAV-APVTRPA
|
tumor cells
|
APVTRPA
|
|
AAV2
|
I-588
|
AAV-DLSNLTR
|
tumor cells
|
DLSNLTR
|
|
AAV2
|
I-588
|
AAV-NQVGSWS
|
tumor cells
|
NQVGSWS
|
|
AAV2
|
I-588
|
AAV-EARVRPP
|
tumor cells
|
EARVRPP
|
|
AAV2
|
I-588
|
AAV-NSVSLYT
|
tumor cells (CML)
|
NSVSLYT
|
|
AAV2
|
I-588
|
AAV-LS1
|
tumor cells (CML), CD34+cells
|
NDVRSAN*
|
|
AAV2
|
I-588
|
AAV-LS2
|
tumor cells (CML), CD34+cells
|
NESRVLS
|
|
AAV2
|
I-588
|
AAV-LS3
|
tumor cells (CML), CD34+cells
|
NRTWEQQ
|
|
AAV2
|
I-588
|
AAV-LS4
|
tumor cells (CML), CD34+cells
|
NSVQSSW
|
|
AAV2
|
I-588
|
AAV-RGDLGLS
|
tumor cells
|
RGDLGLS
|
|
AAV2
|
I-588
|
AAV-RGDMSRE
|
tumor cells
|
RGDMSRE
|
|
AAV2
|
I-588
|
AAV-ESGLSQS
|
tumor cells
|
ESGLSQS
|
|
AAV2
|
I-588
|
AAV-EYRDSSG
|
tumor cells
|
EYRDSSG
|
|
AAV2
|
I-588
|
AAV-DLGSARA
|
tumor cells
|
DLGSARA
|
|
AAV2
|
I-588
|
AAV-GPQGKNS
|
tumor cells
|
GPQGKNS
|
|
AAV2
|
I-588
|
AAV-NSSRDLG
|
endothelial cells
|
NSSRDLG
|
|
AAV2
|
I-588
|
AAV-NDVRAVS
|
endothelial cells
|
NDVRAVS#
|
|
AAV2
|
I-588
|
AAV-PRSTSDP
|
lung (maybe endothelial cells)
|
PRSTSDP
|
|
AAV2
|
I-588
|
AAV-DIIRA
|
endothelial cells
|
DIIRA
|
|
AAV2
|
I-588
|
AAV-SYENV
|
endothelial cells
|
SYENVASRRPEG
|
|
AAV2
|
I-588
|
AAV-PENSV
|
endothelial cells
|
PENSVRRYGLEE
|
|
AAV2
|
I-588
|
AAV-LSLAS
|
endothelial cells
|
LSLASNRPTATS
|
|
AAV2
|
I-588
|
AAV-NDVWN
|
endothelial cells
|
NDVWNRDNSSKRGGTTEAS
|
|
AAV2
|
I-588
|
AAV-NRTYS
|
endothelial cells
|
NRTYSSTSNSTSRSEWDNS
|
|
AAV2
|
I-588
|
rAAV2-ESGHGYF
|
pulmonary endothelial cells
|
ESGHGYF
|
|
AAV2
|
I-588
|
AAV-GQHPRPG
|
cardiomyoblasts
|
GQHPRPG+
|
|
AAV2
|
I-588
|
AAV-PSVSPRP
|
cardiomyoblasts
|
PSVSPRP
|
|
AAV2
|
I-588
|
AAV2-VNSTRLP
|
cardiomyoblasts
|
VNSTRLP
|
|
AAV2
|
I-588
|
AAV-LSPVR
|
cardiomyoblasts
|
LSPVRPG
|
|
AAV2
|
I-588
|
AAV-MSSDP
|
cardiomyoblasts
|
MSSDPRRPPRDG
|
|
AAV2
|
I-588
|
AAV-GARPS
|
cardiomyoblasts
|
GARPSEVTTRPG
|
|
AAV2
|
I-588
|
AAV-GNEVL
|
cardiomyoblasts
|
GNEVLGTKPRAP
|
|
AAV2
|
I-588
|
AAV-KMRPG
|
cardiomyoblasts
|
KMRPGAMGTTGEGTRVTRE
|
|
AAV2
|
I-588
|
AAV588MTP
|
skeleton muscle
|
ASSLNIA
|
Table 1: AAV2 variants with peptide chains inserted at I-587 and I-588
In addition to I-587 and I-588, I-453, I-520&I-584, I-584, and I-585 have also been successfully used to develop new cell targeted engineered AAVs (Table 2).
|
Serotype
|
Position
|
Name
|
Target Cell Type
|
Insert
|
|
AAV2
|
I-453
|
rRGD453ko
|
av integrin-positive tumor cells
|
CDCRGDCFC
|
|
AAV2
|
I-453
|
AAV-MNVRGDL
|
endothelial cells
|
MNVRGDL
|
|
AAV2
|
I-453
|
AAV-ENVRGDL
|
endothelial cells
|
ENVRGDL
|
|
AAV2
|
I-520 and I-584
|
A520/N584 (RGD)
|
av integrin-positive tumor cells
|
CDCRGDCFC
|
|
AAV2
|
I-584
|
A584-RGD4C
|
av integrin-positive tumor cells
|
CDCRGDCFC
|
|
AAV2
|
I-584
|
A584-RGD4CALS
|
av integrin-positive tumor cells
|
CDCRGDCFC
|
|
AAV2
|
I-585
|
AAV-∆IV-NGR
|
CD13-positive tumor cells
|
NGRAHA
|
|
AAV2
|
I-585
|
AAV-PTP
|
Plectin-positive tumor cells
|
KTLLPTP
|
Table 2: Non I-587 and I-588 Shell Variants
As stated in the research background, pre-existing neutralizing antibodies in vivo are a major obstacle limiting the widespread clinical application of AAV. Serological studies have shown that most people have been exposed to wild-type AAV, so neutralizing antibodies against AAV may have already formed in their bodies. These neutralizing antibodies may interfere with AAV entry into target cells, intracellular transport, and unpacking in the nucleus, thereby preventing transduction. According to reports, the prevalence of anti AAV antibodies in the population is about 40-80%. Among them, neutralizing antibodies against AAV2 are relatively more popular in the population. To overcome this obstacle, scientists have developed a series of new engineered AAVs using other low immunogenicity serotypes as scaffolds and referencing the capsid modification sites of AAV2 (Table 3).
|
Serotype
|
Position
|
Name
|
Target Cell Type
|
Insert
|
|
AAV1
|
I-590
|
BAP-AAV1
|
Scavidin displaying BT4C (rat glioma)
|
GLNDIFEAQKIEWHE
|
|
AAV1
|
I-590
|
BAP-AAV1
|
endothelial cells
|
GLNDIFEAQKIEWHE plus CDCRGDCFC(RGD4C)
|
|
AAV1
|
I-590
|
AAV1-RGD
|
tumor cells, endothelial cells
|
CDCRGDCFC
|
|
AAV1
|
I-590
|
AAV1-RGD/BAP (90/10) (mosaic capsid)
|
tumor cells, endothelial cells
|
CDCRGDCFC and GLNDIFEAQKIEWHE
|
|
AAV1
|
I-590
|
Tet1c-AAV1 (mosaic capsid)
|
tetanus toxin GT1b receptor positive cells
|
HLNILSTLWKYR
|
|
AAV1
|
I-590a
|
AAV1.9-3-SKAGRSP
|
fibroblast
|
SKAGRSP
|
|
AAV5
|
I-575
|
BAP-AAV4
|
tumor cells
|
GLNDIFEAQKIEWHE
|
|
AAV6
|
I-585
|
AAV6-RGD
|
tumor cells
|
RGD
|
|
AAV6
|
I-585 plus Y705-731F+T492V
|
AAV6-RGD-Y705-731F+T492V
|
tumor cells
|
RGD
|
|
AAV6
|
I-585plus Y705-731F+T492V+K531E
|
AAV6-RGD-Y705-731F+T492V+K531E
|
tumor cells
|
RGD
|
|
AAV8
|
I-585c
|
AAV2/8-BP2
|
on-bipolar cells
|
PERTAMSLP
|
|
AAV8
|
I-590
|
AAV8-ESGLSOS
|
tumor cells
|
ESGLSOS135
|
|
AAV8
|
I-590
|
AAV8-ASSLNIA
|
heart (weakly improved transduction)
|
ASSLNIA122
|
|
AAV8
|
I-590d
|
AAV8-GQHPRPG
|
heart (weakly improved transduction)
|
GQHPRPG86
|
|
AAV8
|
I-590d
|
AAV8-SEGLKNL
|
liver
|
SEGLKNL
|
|
AAV9
|
I-589
|
AAV-SLRSPPS
|
endothelial cells, smooth muscle cells
|
SLRSPPS
|
|
AAV9
|
I-589
|
AAV-RGDLRVS
|
endothelial cells, smooth muscle cells
|
RGDLRVS
|
|
AAV9
|
I-589d
|
AAV9-NDVRAVS
|
endothelial cells
|
NDVRAVS82
|
|
AAV9
|
I-589d
|
AAV9-ESGLSOS
|
tumor cells (weak targeting)
|
ESGLSOS135
|
|
AAV9
|
I-588
|
AAV-PHP.B
|
CNS
|
TLAVPFK
|
|
AAV9
|
I-588
|
AAV-PHP.A
|
CNS
|
YTLSQGW
|
|
AAV9
|
I-588
|
AAV9-7m8
|
retinal cells
|
LGETTRP80
|
|
AAV9P1
|
not disclosed
|
AAV9P1
|
neuronal progenitor cells
|
RGDLGLS
|
Table 3: Engineering AAV with Non AAV2 Skeleton
If you want to understand the differences in effectiveness among all serotypes targeting the same type of cells, please refer to the corresponding reference in Reference 2.
Weizhen provides fast and ready to use engineered AAV - ensuring successful packaging!
You can choose the AAV Cap plasmid you are interested in from Table 1, and we will deliver the ready to use AAV virus to you within 1-2 weeks. We promise that if the packaging is unsuccessful, there will be no charge, greatly accelerating your research process! In addition, if you are interested in the plasmids in Tables 2 and 3, we can also provide personalized customization to ensure successful packaging!
Consultation and ordering: ① Call the hotline at 400-077-2566; ② Call your regional manager; ③ Add Weizhen customer service consultation and order below!
5. Conclusion
At present, most clinical studies on AAV gene drugs use natural capsids, which can easily become targets for host enzymes, thereby affecting their overall performance. In addition, natural AAV serotypes have tissue affinity, but usually have a broad tendency. Therefore, high doses of the virus must be used to achieve therapeutic effects. Moreover, the packaging capacity of AAV vectors and the existing neutralizing antibodies in most populations further limit the clinical application of AAV. In order to overcome these limitations, scientists have focused their research on engineering AAV to further improve its safety, efficacy, and specificity, thereby promoting the rapid development of gene therapy. In the current research boom of gene therapy, in order to accelerate your scientific research process, we have established an engineered AAV library with the aim of contributing to the development of gene therapy.
6.References
1. Lugin, M. L., et al. (2020). "Synthetically Engineered Adeno-Associated Virus for Efficient, Safe, and Versatile Gene Therapy Applications." ACS Nano 14(11): 14262-14283.
2. Buning, H. and A. Srivastava (2019). "Capsid Modifications for Targeting and Improving the Efficacy of AAV Vectors." Mol Ther Methods Clin Dev 12: 248-265.
3. Pipe, S., et al. (2019). "Clinical Considerations for Capsid Choice in the Development of Liver-Targeted AAV-Based Gene Transfer." Mol Ther Methods Clin Dev 15: 170-178.
4. Tseng, Y. S. and M. Agbandje-McKenna (2014). "Mapping the AAV Capsid Host Antibody Response toward the Development of Second Generation Gene Delivery Vectors." Front Immunol 5: 9.
5. Gurda, B. L., et al. (2013). "Capsid antibodies to different adeno-associated virus serotypes bind common regions." J Virol 87(16): 9111-9124.