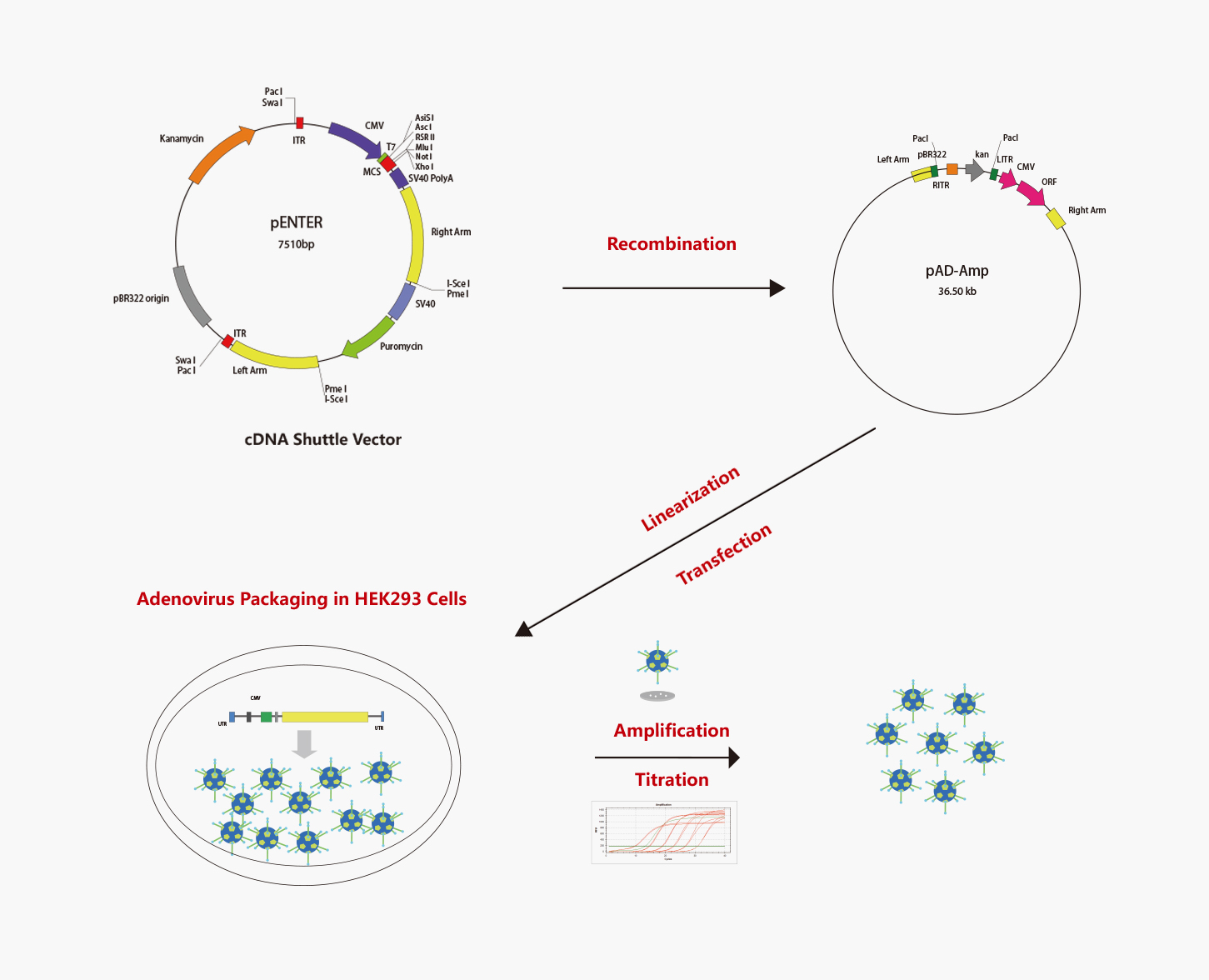
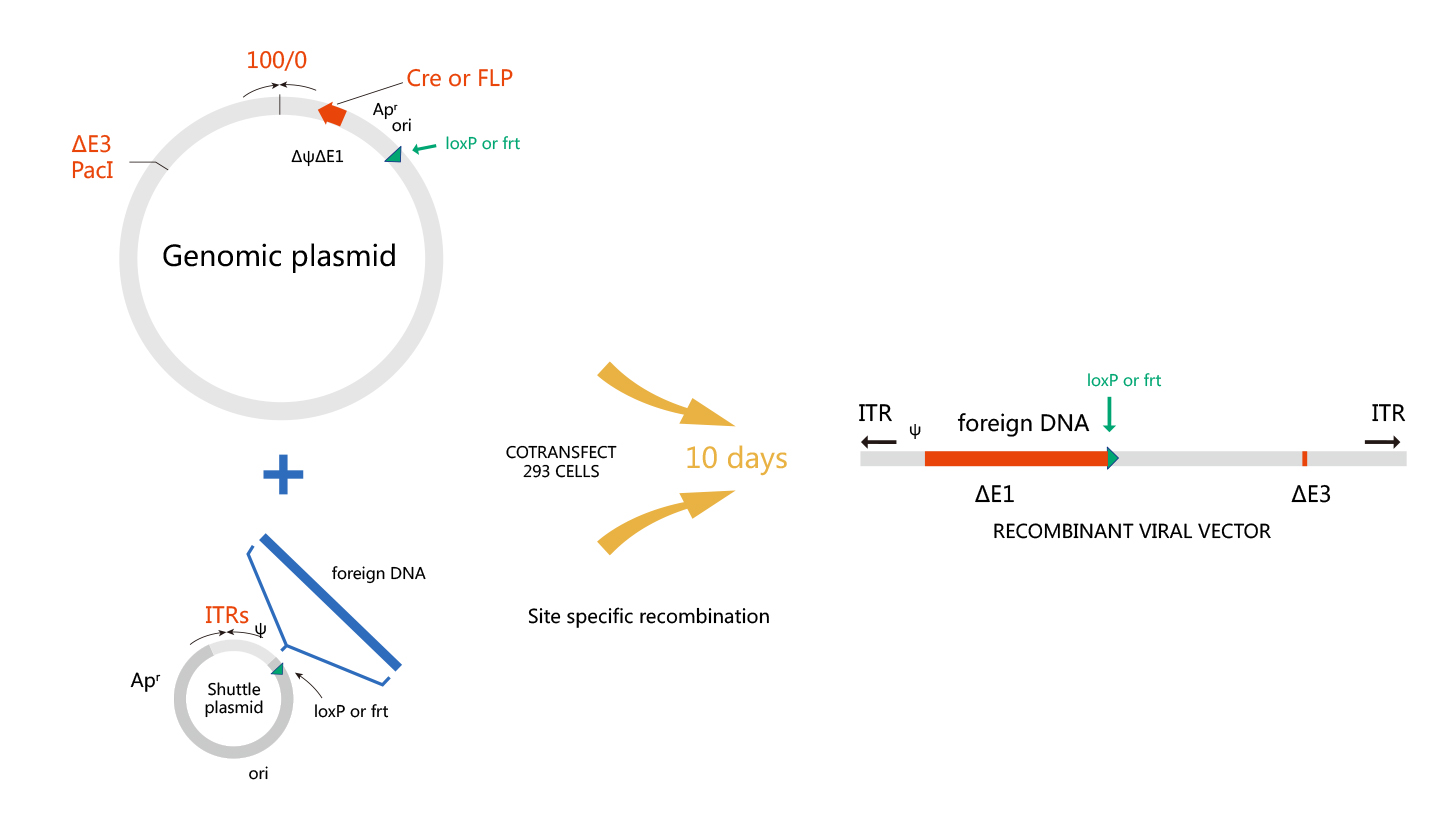
WZ Biosciences uses the AdEasy system and AdMax system to produce recombinant adenoviruses. The AdMax system is a commonly used method for Adenovirus preparation so far due to convenient, high recombination efficiency, and high packaging efficiency. Schematic representation of the AdEasy system and AdMax system are shown respectively in below.
Adenovirus Packaging Process
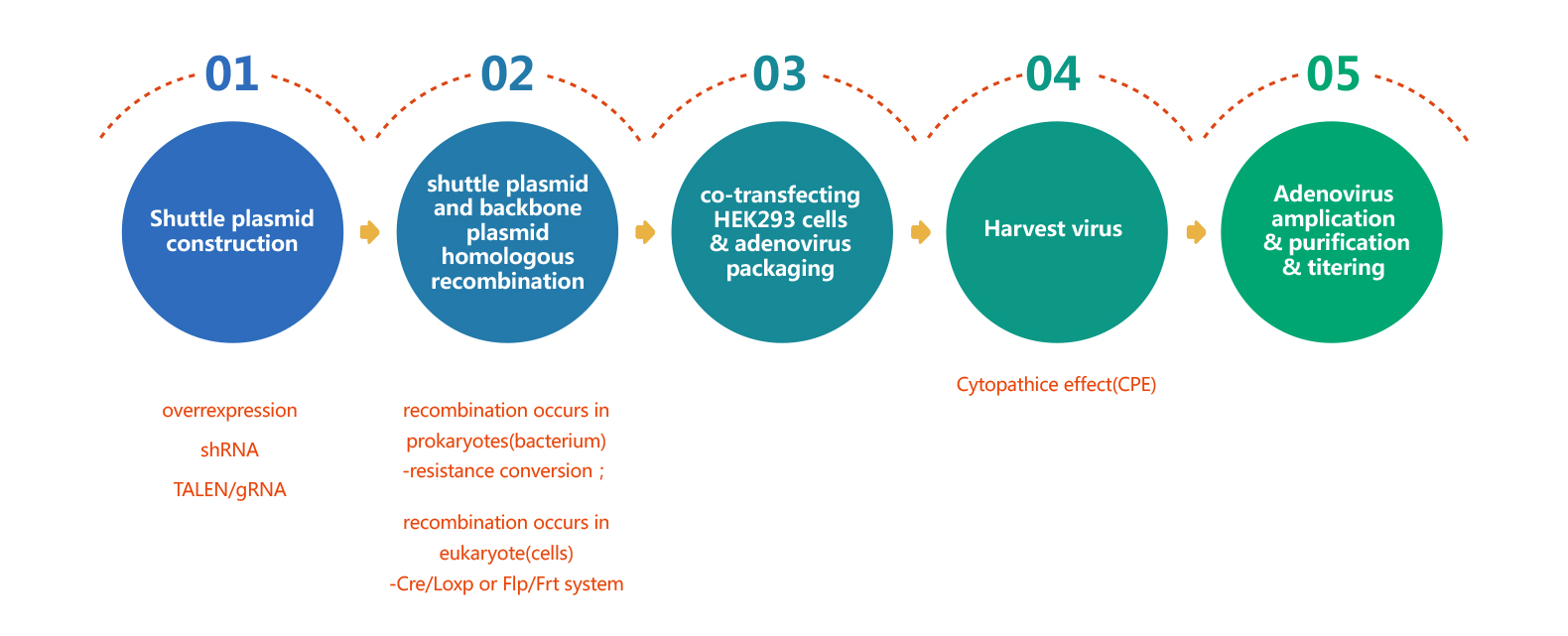
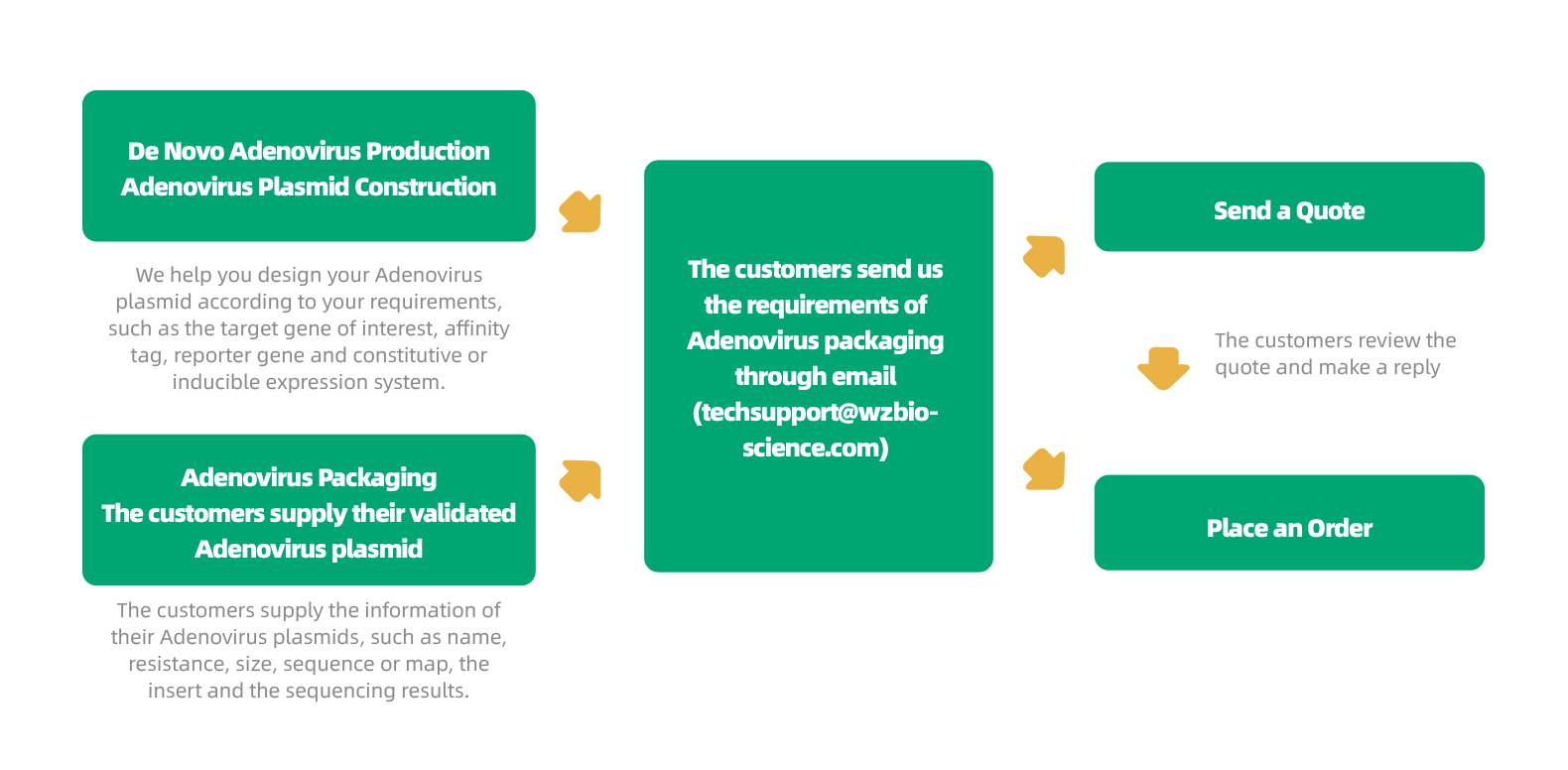
WZ Biosciences offers a full range of services ranging from designing, cloning, packaging to expression analysis. Moreover, WZ Biosciences provides the complete AdV vectors and AdV Expression Systems that can be used to express human/mouse/rat ORFs, lncRNAs, circRNAs, shRNAs, CRISPR/gRNA in vitro and in vivo studies. Working with WZ, you will get high titer, high purity and high stability viral stock. Figure below is the flow chart for AdV production.
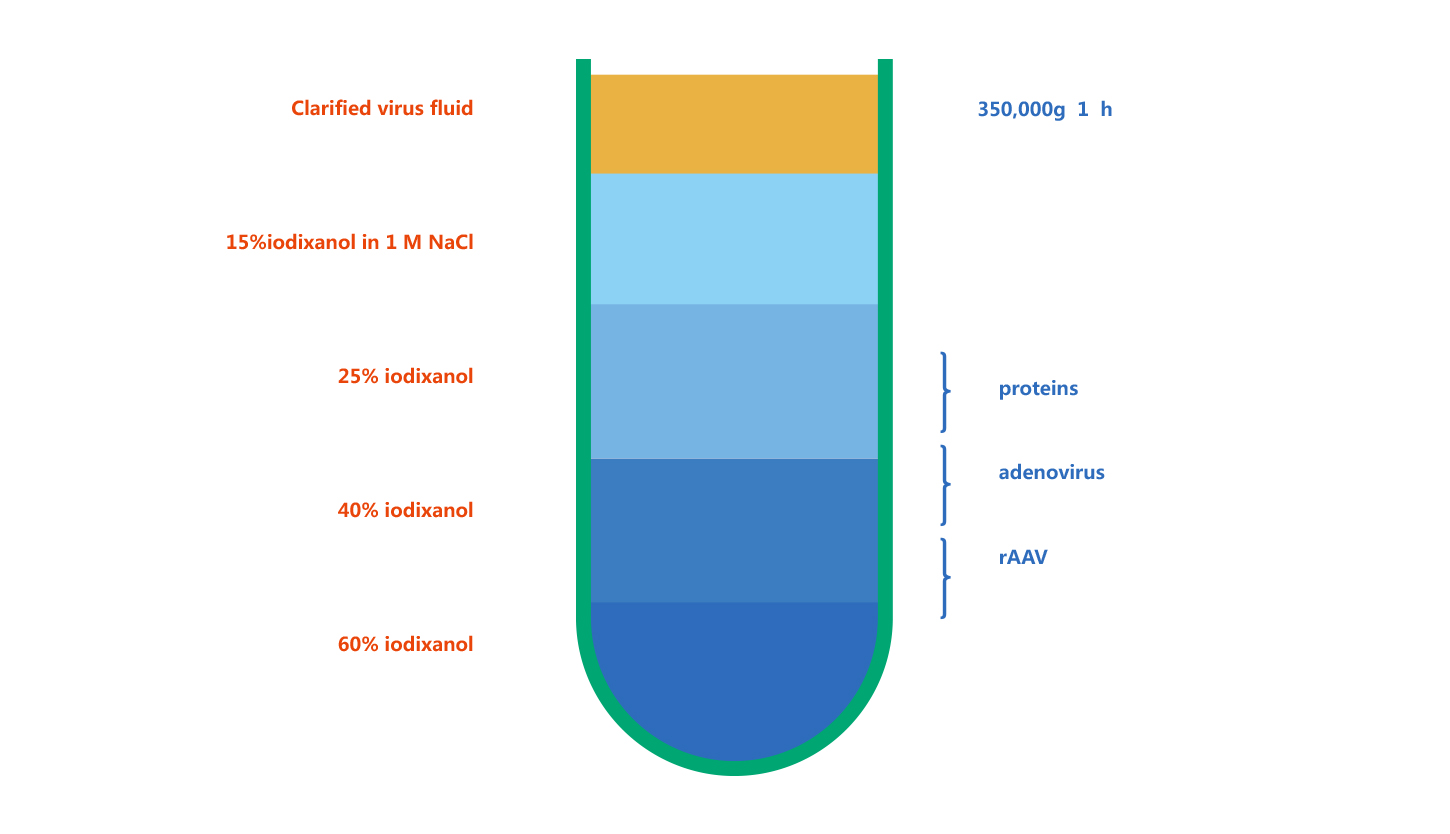
Adenovirus Purification
WZ Biosciences purify adenovirus by Iodixanol Gradient Ultracentrifugation. Iodixanol is a contrast agent, and has been subjected to clinical testing. Iodixanol is non-ionic, non-toxic to cells and metabolically inert. Currently, Iodixanol is widely used for viral purification. Its principle is that particles with different sedimentation rates will lay as different narrow zone under a certain centrifugal force. Figure below is the example of iodixanol gradients before and after centrifugation.
Adenovirus Titering
Multiple methods can be used for quantification of WZ Biosciences adenovirus preparations, including QPCR, Fluorescence Titering Assay and Quick adenovirus titer measurement kit. The following table is the details of the three methods:
|
Methods
|
Principles
|
Titer Units
|
Purposes
|
|
QPCR
|
Performe qPCR assay with specific primers targeting adenovirus genome. The viral titer is quantified by comparison to a standard curve of a plasmid sample of known concentration.
|
vp/ml
(physical titer)
|
Titering adenoviruses from premade adenovirus collection
|
|
Fluorescence Titering Assay
|
Count fluorescent cells
|
pfu/ml
(function titer)
|
Determining adenovirus preparations that carry a fluorescence marker.
|
|
Quick adenovirus titer measurement kit
|
Utilize an antibody against adenovirus hexon proteins to visualize infected cells by immunocytochemistry staining.
|
pfu/ml
(function titer)
|
Measuring all adenovirus preparations, and not limited by fluorescence marker
|
|
|
Adenovirus Structure
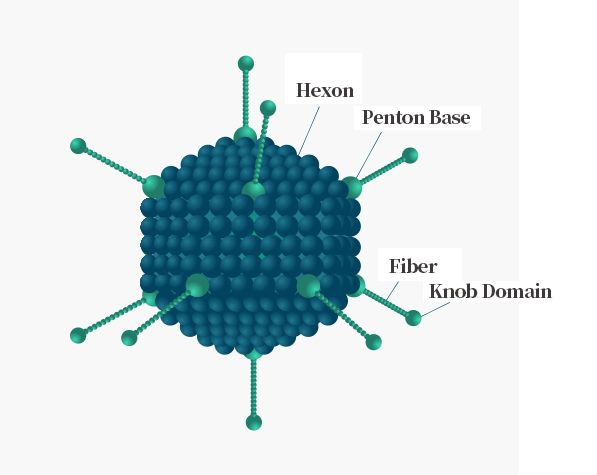
Adenovirus is medium-sized (90–100 nm) virus with an icosahedral nucleocapsid containing 240 trimeric hexons and 12 pentameric penton bases, each of which is bound with one fiber trimer, its distal ends have a globular knob domain. The adenovirus infection cycle starts with attachment to cell surface receptors by the fiber knob domain. The structure of Adenovirus is shown on the right.
|
|
Adenovirus Genome
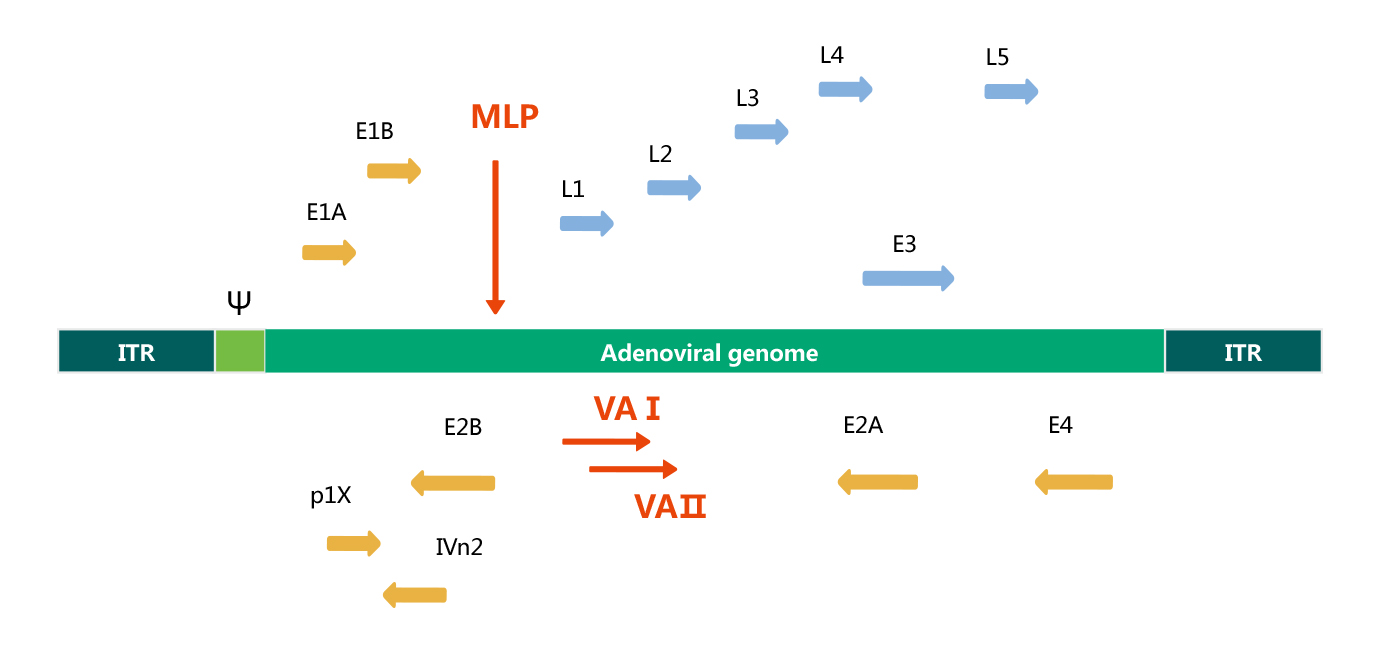
Adenovirus is a linear, double strand DNA virus, the length of its genome is 36 Kb. The Adenovirus genome is flanked by inverted terminal repeats(ITRs), which are required for viral DNA replication, and near the left ITR is a packaging signal (Ψ), which is required for the encapsidation of the Adenovirus genome. The Adenovirus genome is comprised of two main regions: early (E) and late (L) gene. Early genes (E1 to E4) are necessary for viral replication; whereas, late genes(L1 to L5) are required for viral assembly. See the transcription map of human Adenovirus serotype 5 below.
Base on the genome structure and function of human adenovirus serotype 5, recombinant adenovirus vector is generated by removing both E1 and E3 regions. Although the E1 gene is essential for adenovirus packaging, it can be provided in trans in HEK293 packaging cells, that are E1-expressing cells, whereas, the E3 region counteracts host defense mechanism, it is not essential for in vitro viral growth. Rcombinant adenovirus without E1 and E3 genes is able to accommodate larger recombinant genes (up to 7.5 Kb).
Process of Adenovirus Infection
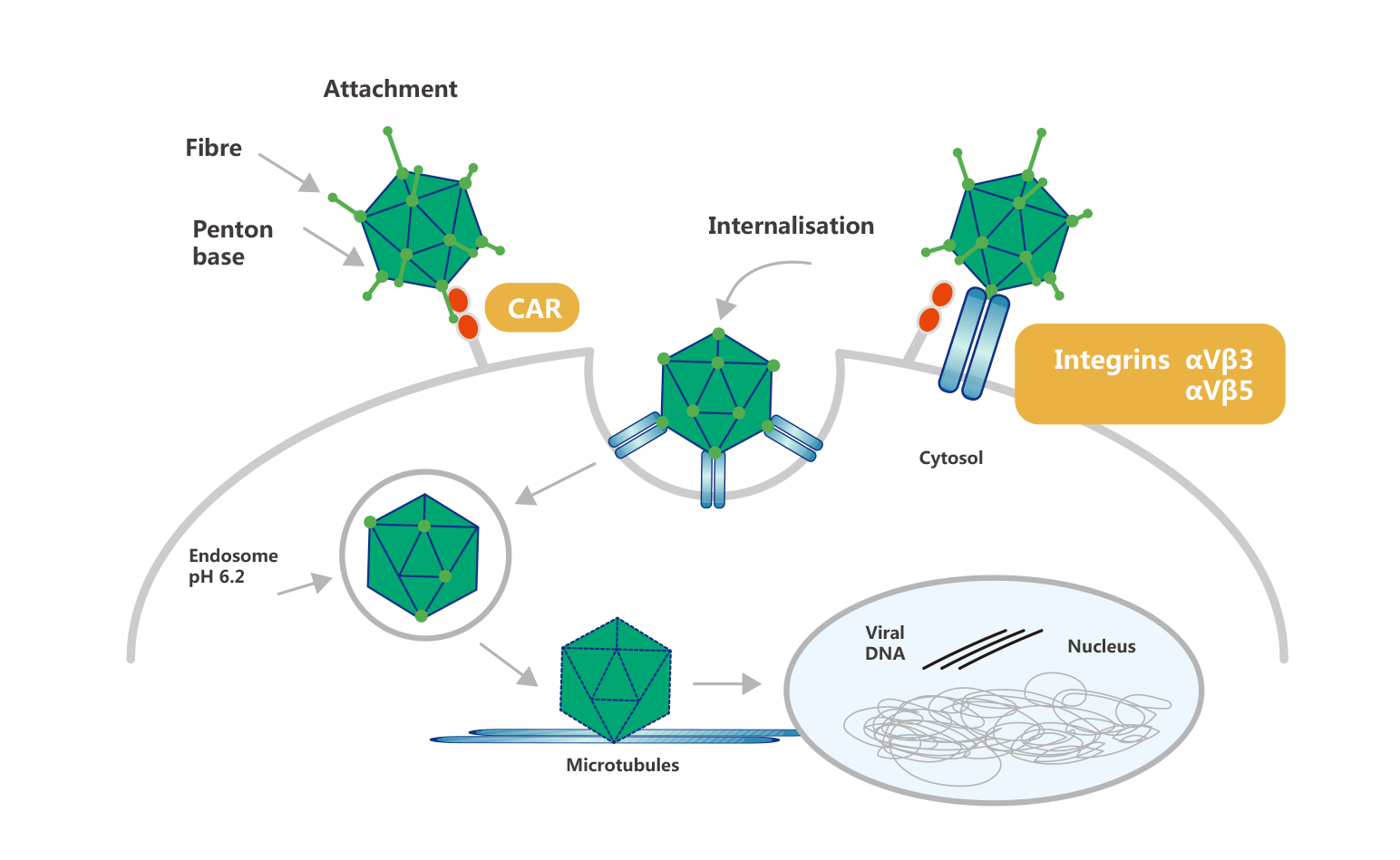
Adenovirus infection starts with the recognition and binding of the distal knob domain of the viral fiber to the adenovirus receptor (coxsackie and adenovirus receptor, CAR). Following CAR binding, the penton base engages integrins αvβ3 and αvβ5 to initiate endocytosis and viral entry. This process is facilitated by the RGD motif in the penton base. The process of Adenovirus infection is shown below:
Characterization of Recombinant Advenovirus
1. The adenovirus has a very broad host range. It can infect a wide range of cells including dividing and non-dividing mammalian cells. Some lymphoid cell lines are excepted, because they are resistant to adenoviral infection.
2. Adenovirus has high infection efficiency. It is able to deliver genes with 100% efficiency under optimal infection conditions.
3. Adenovirus can easily obtain very high titer, and concentrated to 1012-13VP/ml(1010-11pfu/ml).
4. Adenovirus is easily amplified in HEK293 cells compared with other viruses such as lentivirus and adeno-associated virus which are need to be repackaged.
5. Adenovirus genome rarely integrate into the host chromosome and does not inactivate genes or activate oncogenes.
6. Adenovirus is relatively stable. It can be stored at fridge for weeks, -20◦C freezers for months and -80◦C freezers for years.
Ad5 & Ad5/F35
The efficiency of Ad5 infection depends on CAR, and av integrins(αvβ3 & αvβ5) density. However, some immune cells express scarcely CAR and av integrins, such as hemopoietic stem cells. Therefore, numerous studies focused on the development of a new adenovirus serotype. Finally, serotype 35 emerged as the variant with the highest tropism for hemopoietic stem cells. Therefore, a chimeric vector (Ad5/F35) was developed by scientists, which contained the short shafted Ad35 fiber incorporated into an Ad5 capsid. The structure and difference of Ad5 & Ad5/F35 are given in the below.
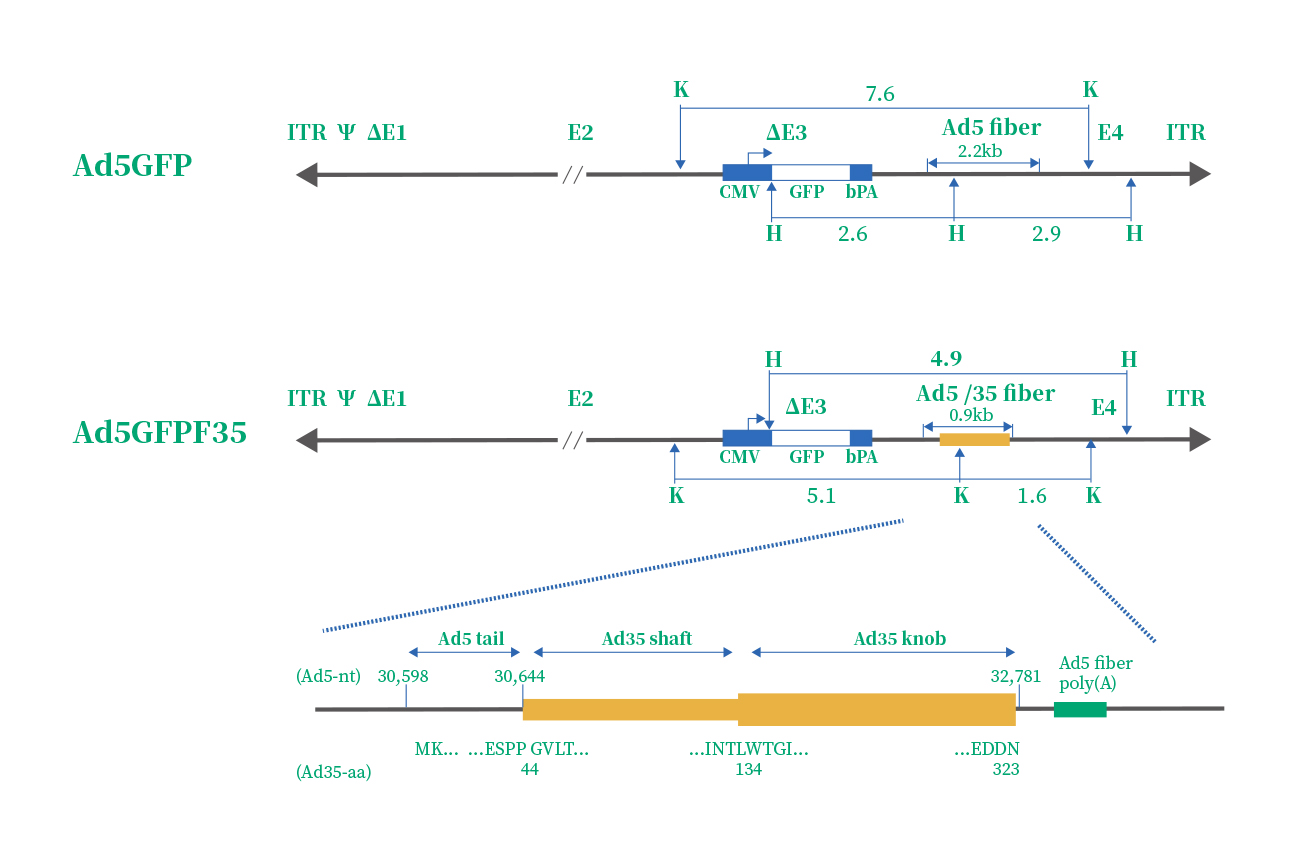
|
|
Adv Serotypes
|
Vector Characteristics
|
Function
|
Cell Receptors
|
Package System
|
|
AdV5
|
Recombinant adenovirus vectors (E1/E3 deleted) are able to accommodate recombinant genes (up to 8kb)
|
Infect most cell types (except stem cells and cancer cells)
|
CAR
|
AdMax
|
|
AdV5/F35
|
The chimeric vectors (Adv5/F35) are developed from Adv5 and contains the fiber domain (Knob and sharft) derived from Adv35. Since the adenoviral receptor is CD46, Adv5/F35 has ability to highly infect cells that CAR expression is limited, such as stem cells and cancer cells.
|
Infect all nucleated cells in theory
|
CD46
|
AdMax
|
|
1. Complete vectors:
|
More than 20 different destination vectors available for different fluorescent and affinity tags at either N-terminal or C-terminal.
|
|
|
|
2. Quick delivery:
|
All adenovirus cDNA & mirRNA cloning vectors are compatible with our Entry & MIR clones MCS–choose from 18,000 ORF clones and 1300 miroRNA clones.
|
|
|
|
3. High Purify:
|
Purified adenoviruses by modified Iodixanol Gradient Ultracentrifugation are ready to use in vivo studies.
|
|
|
|
4. Excellent service:
|
Based on your research goal, our professional technical staffs will design experimental program and manufacture the most ideal adenovirus particles for you.
|

|
《Adenovirus technical manual》
|
|
|
1. Hello, the cells I infected are trophoblast cells. 100,000 cells were inoculated in a six-well plate firstly, then virus (MOI=40) was added after 16 hours of attachment. 30 hours later, the cells became full. I would like to ask: After virus infection, the cell production of the experimental group is similar to that of the control group. Is it normal?
If the state of cells infected with adenovirus is not significantly different from that of the uninfected group, it means that the virus has no obvious toxic effect on cells. Therefore, it may not be that the number of viruses is insufficient. Non-adenovirus packaging cells will not have any obvious characteristics after being infected by adenovirus. If you add the virus to the culture dish after diluting the virus with the medium in advance when adding the virus, then the high MOI value (causing the cells to float) is relatively high for the target cells. Therefore, don’t worry, you can first check whether there is protein expression.
2. Is it necessary to replace the fresh culture medium after adenovirus infects cells?
Whether it is necessary to replace the fresh culture medium depends on the amount of virus added. When the amount of virus is large, it will be toxic to the cells: if there is a change in the cell state during the microscopic examination, the fresh medium needs to be replaced in about 4-8 hours; If there is no obvious change in the cell state, there is no need to replace the fresh culture medium after the virus infects the cell. Or, if you are worried that the virus will be toxic after acting on the cells for a long time, you can also replace the fresh culture medium after 12 hours.
3. Why the cells are all floating after 4-6 hours of adenovirus infection?
Three main reasons can explain the phenomenon above:
1) Cell status: The cells plated before infection must be healthy cells;
Improper operation: When adding virus, if the local virus concentration is too high, it will cause cell death. Therefore, we recommend mixing the virus and medium before adding.
If the 2 points above are both correct, then the amount of virus added is too large.
4. What is the basis for the amount of adenovirus virus when infection the cells?
Selecting the optimal virus amount is the key for getting the best experimental results.
Insufficient amount can’t reach 100% infection efficiency; too high amount will cause toxicity to cells. So how to determine it? Multiplicity of infection (MOI) plays a decisive role. MOI is the ratio of virus to infection cell number. The number of adenovirus receptors on cell surface is different in different cell lines, which determines that the MOI of different cell lines will be different. Generally, for susceptible cell lines, the MOI range used is 10-100. However, for some hard-to-infect cell lines, MOI may need to be as high as 1,000. For most cell lines, the optimal MOI range is very narrow. We recommend you to read the relative literature, or to test the best MOI by infecting the target cells with the adenovirus of the reporter gene before the formal experiment.
Calculation formula for optimal virus dosage
The amount of virus pfu=optimal MOI *cell number/ virus titer
For example, if the optimal MOI of target cell is MOI=10, 106 cells will be infected, then 107pfu virus are needed. If the virus titer is 1×1010 pfu/mL, then 1ul will be used for the experiment.
5. How long can the expression of adenovirus be detected at the gene level and protein level after infecting cells? Is there a difference in the detection time between secreted proteins and non-secreted protein?
The target gene carried by adenovirus expresses quickly. If you want to detect gene expression at the gene level, then perform the test between 12-24 hours of virus-infected cells; if you want to detect the gene expression at protein level, then perform the test between 48-72 hours of virus-infected cells.
For secreted protein and non-secreted protein, the detection time is the same. If you worried about protein secretion, you can do western blot together with the medium.
6. The adenovirus seed is contaminated by bacteria, there is no extra virus seed, and there is no constructed vector, what should I do?
The diameter of adenovirus is 90-100nm, and the diameter of bacteria is much larger, so it can be filtered with a 22um filter. If your virus load is very low and you are worried that you will lose too much after filtering and cannot proceed with the subsequent amplification work, you can directly amplify the contaminated adenovirus firstly. Of course, adenovirus contaminated with bacteria are obtained after amplification. But the amount of virus is increased, then use a 22um filter for filtration.
7. How to identify the target gene that adenovirus delivered?
To identify as follow:
①Design primers for genes, which can also be used as amplification and sequencing;
②Virus is the template which is treated with proteinase K. If it is an unpurified virus, it needs to pass through a purification column to remove the medium components.
③Perform PCR with the processed virus as a template;
④Sequencing the PCR products with PCR primers.
Premade Human ORF Adenovirus:
|
1.
|
Biol Psychiatry. (IF=12.095). Zhao D, et al. (2019). RPS23RG1 Is Required for Synaptic Integrity and Rescues Alzheimer's Disease-Associated Cognitive Deficits.
|
|
|
2.
|
J Clin Endocrinol Metab. (IF=5.399). Li M, et al. (2019). The HMGA2-IMP2 Pathway Promotes Granulosa Cell Proliferation in Polycystic Ovary Syndrome.
|
|
|
3.
|
Int J Cardiol. (IF=3.229). Wu B, et al. (2018). Mesoderm/mesenchyme homeobox gene l promotes vascular smooth muscle cell phenotypic modulation and vascular remodeling.
|
|
|
4.
|
Life Sci. (IF=6.304). He M, et al. (2018). In vitro study of FUZ as a novel potential therapeutic target in non-small-cell lung cancer.
|
|
|
5.
|
Cell Death Dis. (IF=6.304). Sun S, et al. (2017). Loss of the novel mitochondrial protein FAM210B promotes metastasis via PDK4-dependent metabolic reprogramming.
|
|
|
6.
|
Molecular Medicine Reports. (IF=2.1). Zhang S, et al. (2017). PAR1-mediated c-Jun activation promotes heat stress-induced early stage apoptosis of human umbilical vein endothelial cells.
|
|
|
7.
|
Hepatology. (IF=14.679). Xiang D, et al. (2017). Shp2 promotes liver cancer stem cell expansion by augmenting β-atenin signaling and predicts chemotherapeutic response of patients.
|
|
|
8.
|
Cancer Letters. (IF=7.36). Wang Z , et al . (2017). CXCL1 from tumor-associated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrinβ1/FAK/AKT signaling.
|
|
|
9.
|
Gene. (IF=2.984). Zhang J W, et al. (2017). Validation of aspirin response-related transcripts in patients with coronary artery disease and preliminary investigation on CMTM5 function.
|
|
|
10.
|
Cell Death Dis. (IF=6.304). Zheng Y, et al. (2017). Berbamine postconditioning protects the heart from ischemia/reperfusion injury through modulation of autophagy.
|
|
|
11.
|
Oncoimmunology. (IF=5.869). Wang H, et al. (2016). Interactions between colon cancer cells and tumor-infiltrated macrophages depending on cancer cell-derived colony stimulating factor 1.
|
|
|
12.
|
Basic Res Cardiol. (IF=11.981). Sun Z, et al. (2016). Cross-talk between macrophages and atrial myocytes in atrial fibrillation.
|
|
|
13.
|
Mol Carcinog. (IF=3.825). Wang J, et al. (2016). Endothelial cell-anchored tissue factor pathway inhibitor regulates tumor metastasis to the lung in mice.
|
|
Premade Human microRNA Adenovirus:
|
1.
|
Stem Cells Int. (IF=3.869). Li X, et al. (2019). MicroRNA-150 Modulates Adipogenic Differentiation of Adipose-Derived Stem Cells by Targeting Notch3.
|
|
|
2.
|
Oncotarget. (IF=5.168). Tian S, et al. (2017). miR-138-5p suppresses autophagy in pancreatic cancer by targeting SIRT1.
|
|
|
1.
|
Elife Tool. (IF=7.08). Wu L, et al. (2019). PARIS, an optogenetic method for functionally mapping gap junctions.
|
|
|
2.
|
Cell Death Dis. (IF=6.304). Su Y, et al. (2019). MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis.
|
|
|
3.
|
EBioMedicine. (IF=5.736). Wang K, et al. (2019). TGF-beta1/p65/MAT2A pathway regulates liver fibrogenesis via intracellular SAM.
|
|
|
4.
|
Arthritis Res Ther. (IF=4.103). Zeng J, et al. (2019). Interferon-alpha exacerbates neuropsychiatric phenotypes in lupus-prone mice.
|
|
|
5.
|
Oncogene. (IF=7.971). Yu M, et al. (2018). Circadian regulator NR1D2 regulates glioblastoma cell proliferation and motility.
|
|
|
6.
|
Cell Death Discov. (IF=4.114). Zhang SQ, et al. (2018). Oleanolic acid enhances neural stem cell migration, proliferation, and differentiation in vitro by inhibiting GSK3beta activity.
|
|
|
7.
|
Int J Mol Med. (IF=3.098). Zhang L, et al. (2018). Anti-inflammatory effects of Lefty-1 in renal tubulointerstitial inflammation via regulation of the NF-kappaB pathway.
|
|
|
8.
|
Redox Biol. (IF=9.986). Jin Q, et al. (2018). DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways.
|
|
|
9.
|
Cell Physiol Biochem. (IF=5.5). Wang Y, et al. (2018). Inhibition of Histone Deacetylases Prevents Cardiac Remodeling After Myocardial Infarction by Restoring Autophagosome Processing in Cardiac Fibroblasts.
|
|
|
10.
|
Brain,Behavior,and Immunity. (IF=6.633). Zhou Y.T, et al. (2017). Interleukin-1βimpedes oligodendrocyte progenitor cell recruitment and white matter repair following chronic cerebral hypoperfusion.
|
|
|
11.
|
J Pharmacol Sci. (IF=2.835). Li J, et al. (2016). PKCζ interacts with STAT3 and promotes its activation in cardiomyocyte hypertrophy.
|
|
|
12.
|
Arthritis Rheumatol. (IF=9.586). Han X, et al. (2016). MicroRNA-130b Ameliorates Murine Lupus Nephritis Through Targeting the Type I Interferon Pathway on Renal Mesangial Cells.
|
|
|
13.
|
Sci Rep. (IF=3.998). Zhang H, et al. (2016). Endogenous sulfur dioxide is a novel adipocyte-derived inflammatory inhibitor. entific Reports.
|
|
|
14.
|
Mol Cell Biol. (IF=3.611). Wu C, et al. (2016). Phosphatidylinositol 3-Kinase/Akt Mediates Integrin Signaling To Control RNA Polymerase I Transcriptional Activity.
|
|
|
1.
|
Journal of Cellular and Molecular Medicine. (IF=4.486). Wang, et al. (2020). Inhibition of PFKFB3 suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss.
|
|
|
2.
|
EBioMedicine. (IF=5.736. Li, et al. (2019). Inhibition of AZIN2-sv induces neovascularization and improves prognosis after myocardial infarction by blocking ubiquitin-dependent talin1 degradation and activating the Akt pathway.
|
|
|
3.
|
Bone. (IF=4.147). Zhao, et al. (2018). YAP1 is essential for osteoclastogenesis through a TEADs-dependent mechanism.
|
|
|
4.
|
Cardiovascular Research. (IF=8.168. Li, et al. (2018). Loss of AZIN2 splice variant facilitates
endogenous cardiac regeneration.
|
|
|
5.
|
Br J Pharmacol. (IF=6.304). Ma Z.G, et al. (2016). Protection against cardiac hypertrophy by geniposide involves the GLP-1 receptor / AMPKalpha signalling pathway.
|
|
|
6.
|
Clin Sci . (IF=5.223). Ling Y, et al. (2016). Polydatin post-treatment alleviates myocardial ischaemia/reperfusion injury by promoting autophagic flux.
|
|
|
7.
|
Int J Biol Sci. (IF=4.858). Ma Z G, et al. (2016). Asiatic Acid Protects against Cardiac Hypertrophy through Activating AMPKalpha Signalling Pathway.
|
|
|
1.
|
Int. J. Mol. Sci. (IF=4.556). Xu, et al. (2020). Effective MSTN Gene Knockout by AdV-Delivered CRISPR/Cas9 in Postnatal Chick Leg Muscle.
|
|