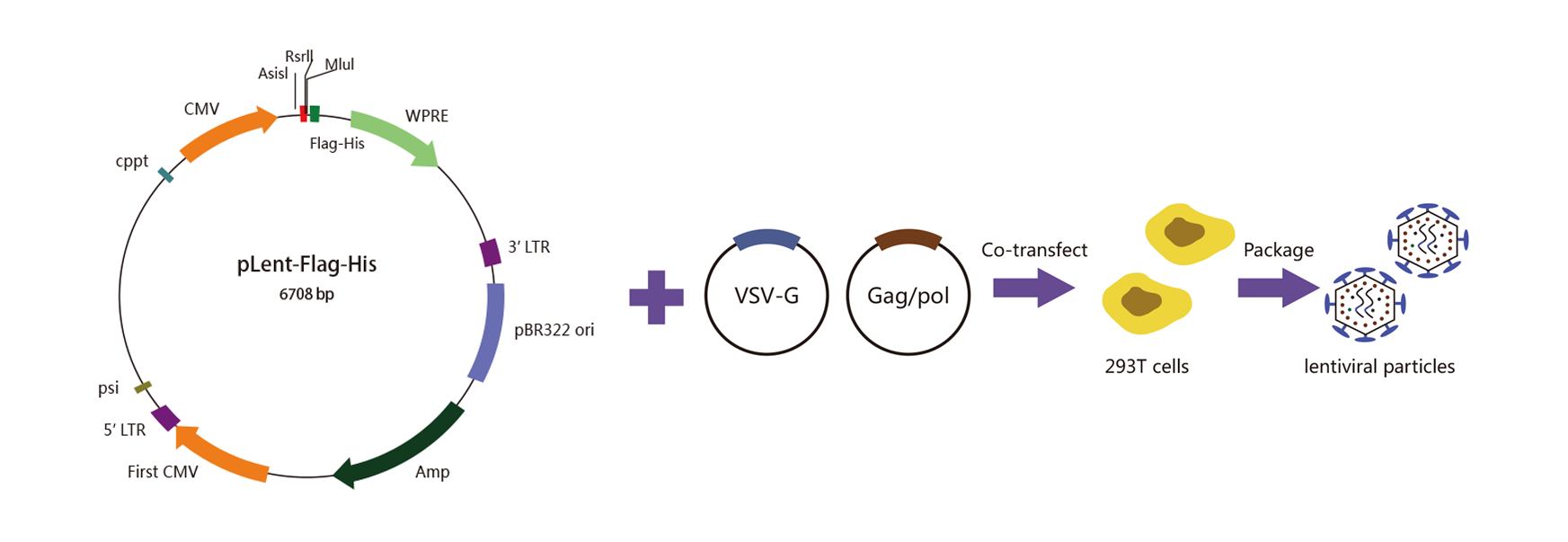
WZ Biosciences produce lentivirus in HEK293T cells using a 2nd Generation lentiviral system, also known as three plasmids co-transfection system. It consists of a packaging plasmid (psPAX2), an envelope expressing plasmid (pMD2G), a lentivirus LTR-containing plasmid carrying multiple clone sites, which can be cloned into a transgene and HEK293T packaging cell lines. The 2nd generation lentivirus packaging system is shown below.
Lentivirus packaging Process
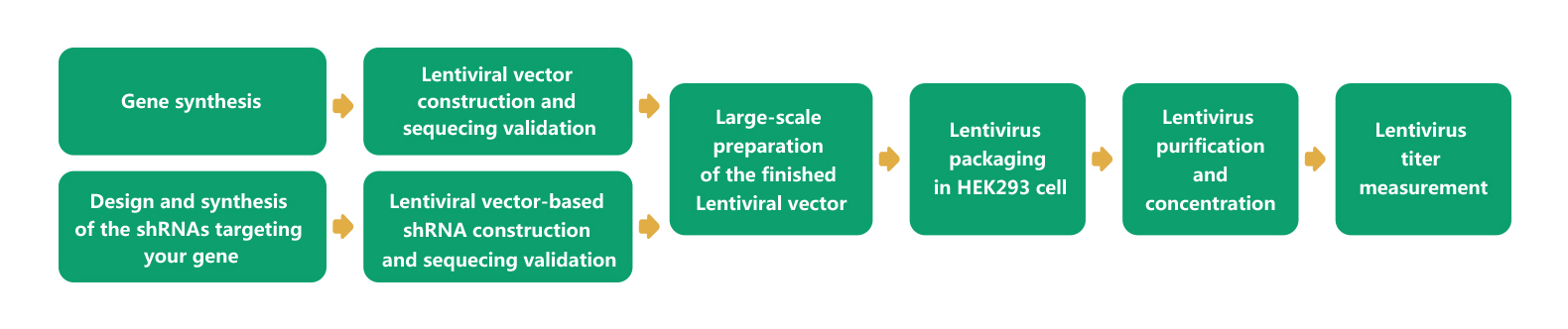
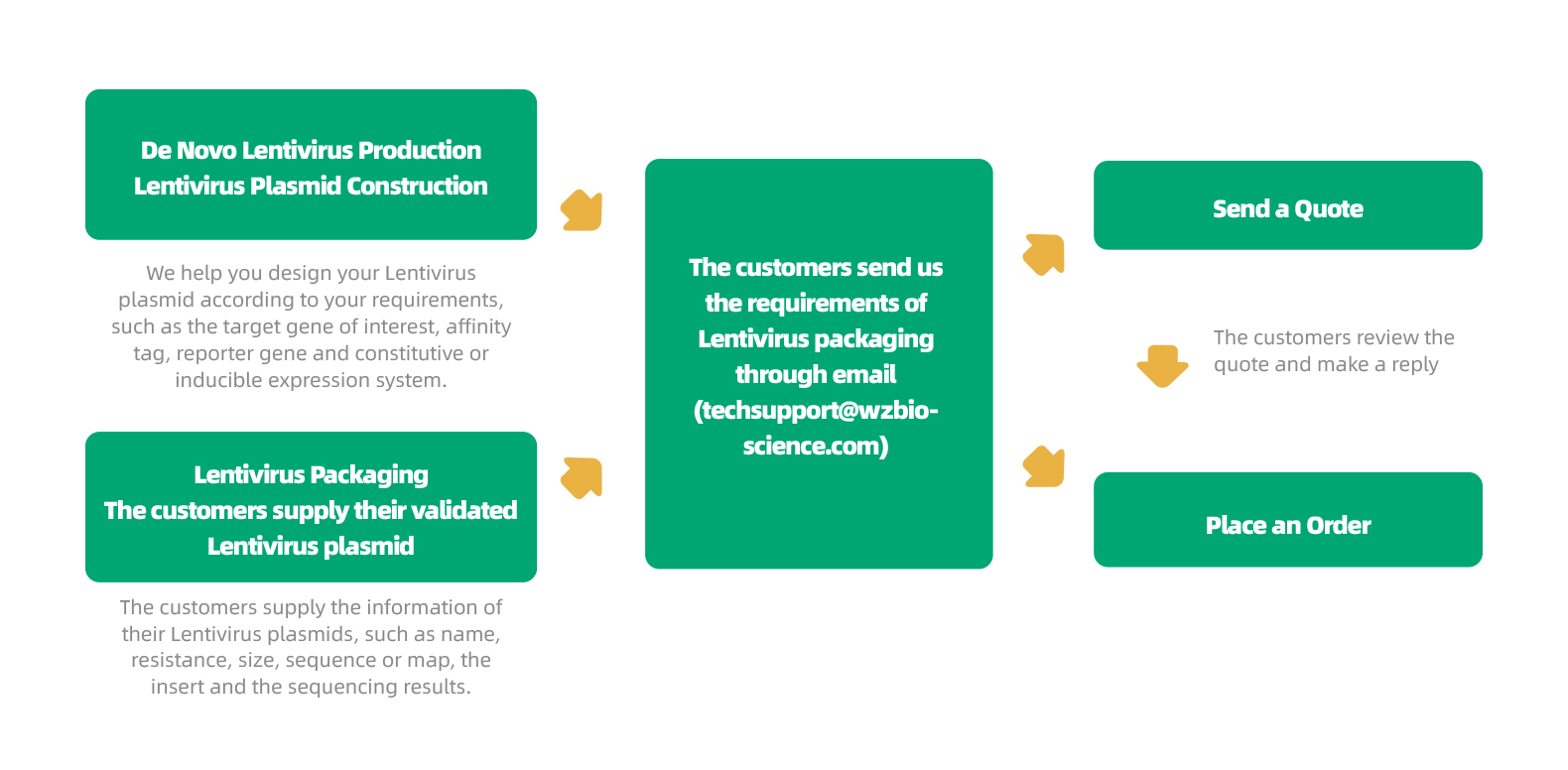
WZ Biosciences provides a complete service from gene cloning into lentiviral vector to lentivirus production. Also, WZ Biosciences offers the complete Lentivirus vectors and Lentivirus Expression Systems that can be used to express human/mouse/rat ORFs, lncRNAs, circRNAs, shRNAs, CRISPR/gRNA in vitro and in vivo studies. The lentivirus production flow chart is in the follow.
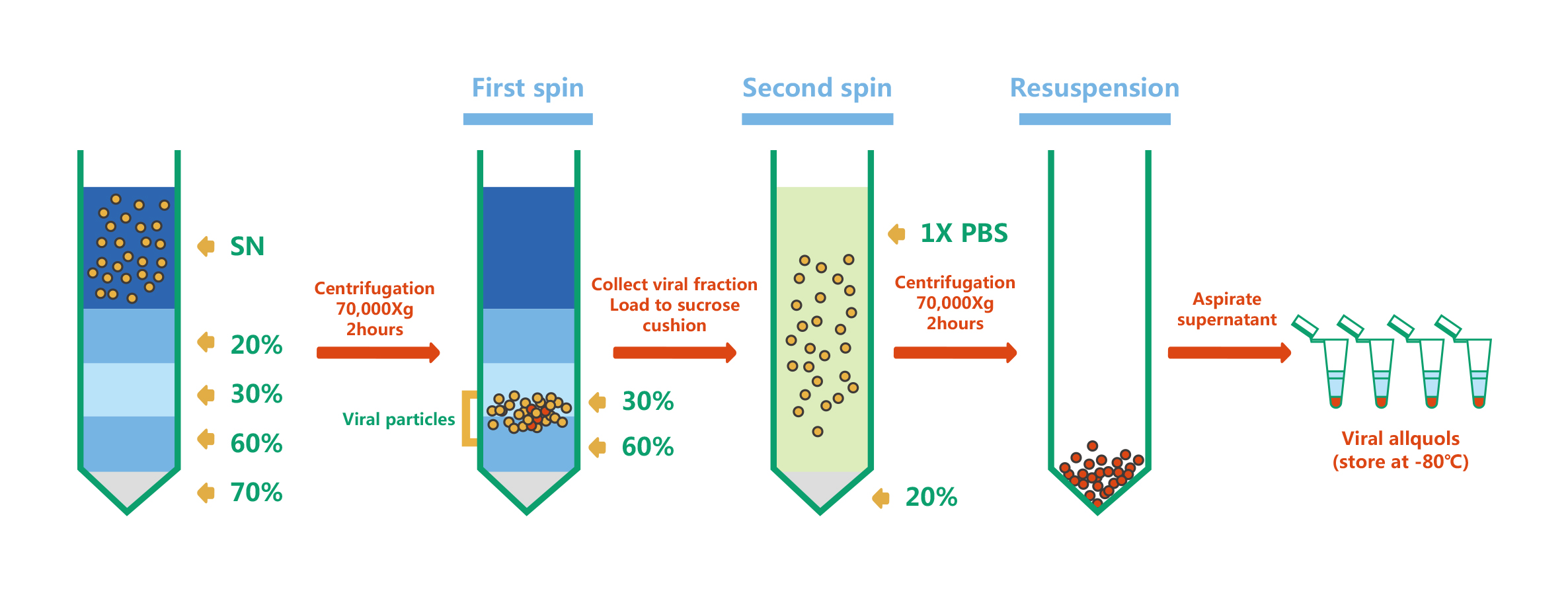
Lentivirus purification
For Lentivirus purification, WZ Biosciences uses sucrose gradient ultracentrifugation. The principle is that particles with different sedimentation rates will lay as different narrow zone under a certain centrifugal force. The lentivirus particles purification and concentration have been shown as follows:
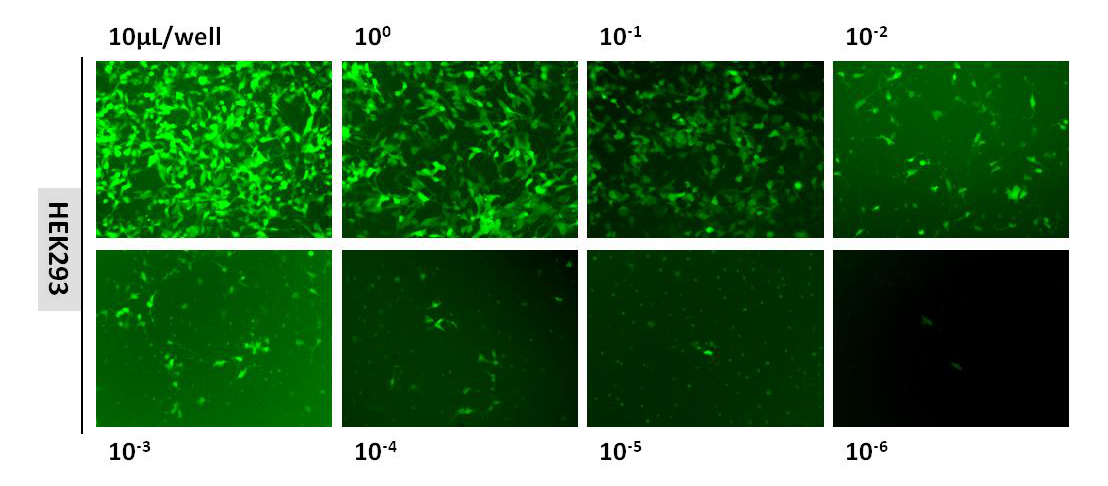
Lentivirus Titering
The estimation of lentiviral titer is performed using Fluorescence Titering Assay. After lentiviral transduction, the infectious lentivirus was determined by counting the number of the fluorescent positive cells using fluorescent microscopy. The figure below is fluorescent images taken using our lentivirus (3.20×108TU/mL) :
|
|
Lentivirus structure
Lentivirus is an enveloped, icosahedral and RNA virus with a diameter of about 80-120 nm. It is spherical in shape. The viral particle is surrounded by an outer protein layer comprised of surface envelop protein (SU) and transmembrane envelope protein (TM), which determines lentiviral tropism, next to the protein layer is matrix protein and capsid. The inner core of viral particle contains two copies of positive-stranded RNA and enzymatic proteins, including reverse transcriptase (RT), integrase (IN), and protease (PR). Schematic of the lentivirus structure is given in right.
|
|
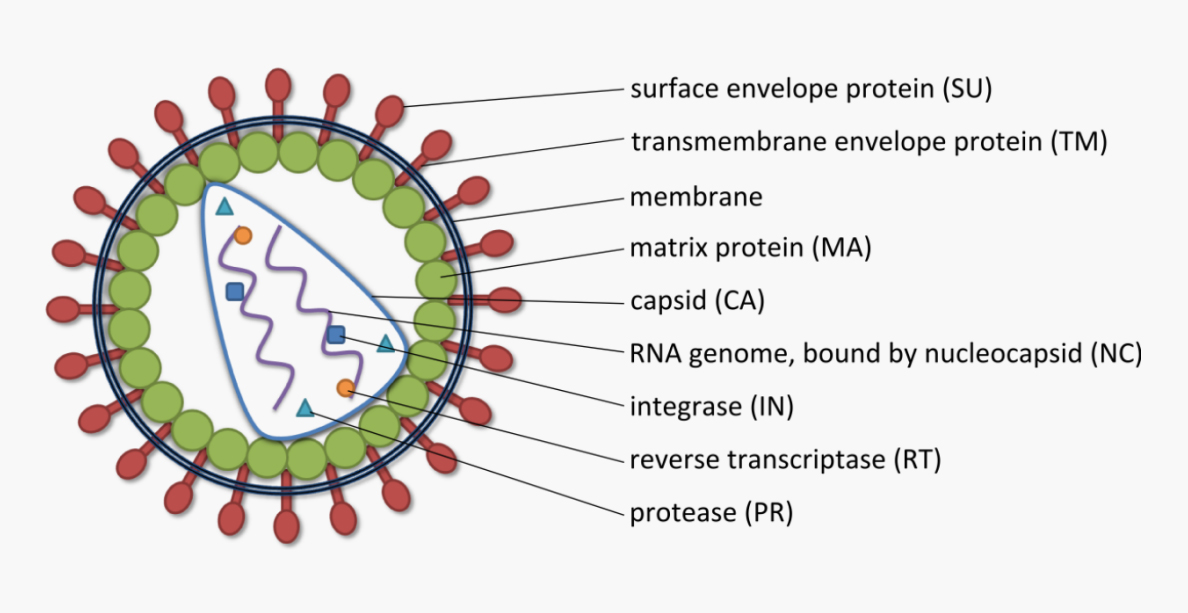
|
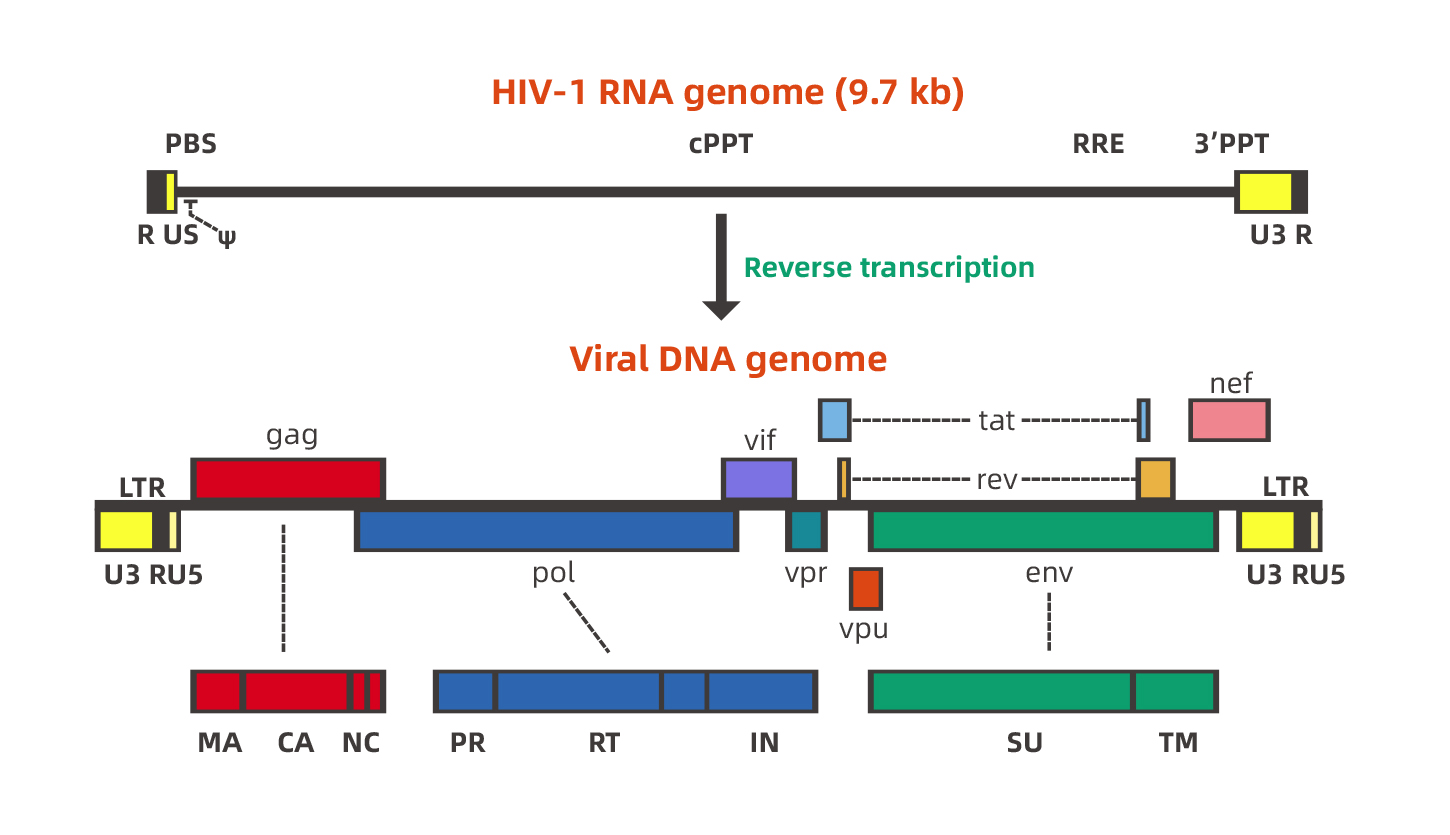
Lentivirus Genome
Lentivirus has a complex genome. Take HIV-1 (Human Immunodeficiency Virus Type I) as an example, HIV is a single-stranded RNA virus with a genome length of approximately 9.7 kb. Two inverted terminal repeats (ITRs) flank the viral genome, and other important genes are in the middle of genome, including three structural genes gag, pol, env and tat, rev, nef, vif, vpr, vpu six regulatory genes. Schematic of the lentivirus genome is given in the follow.
HIV-1-based lentivirus is developed as an excellent gene therapy vector. The research on lentiviral vectors has developed rapidly, and the research is also very in-depth. To date, multiple generation of lentivirus vectors have been designed to increase biosafety and package capability, and the 2nd generation and the 3rd generation recombinant lentiviral vector system are commonly used. Although the package capability is 7-8kb, the lentivirus titer will be infected when the foreign fragment is larger than 3kb. The figure below represents the 2nd generation and 3rd generation recombinant lentiviral vectors separately.
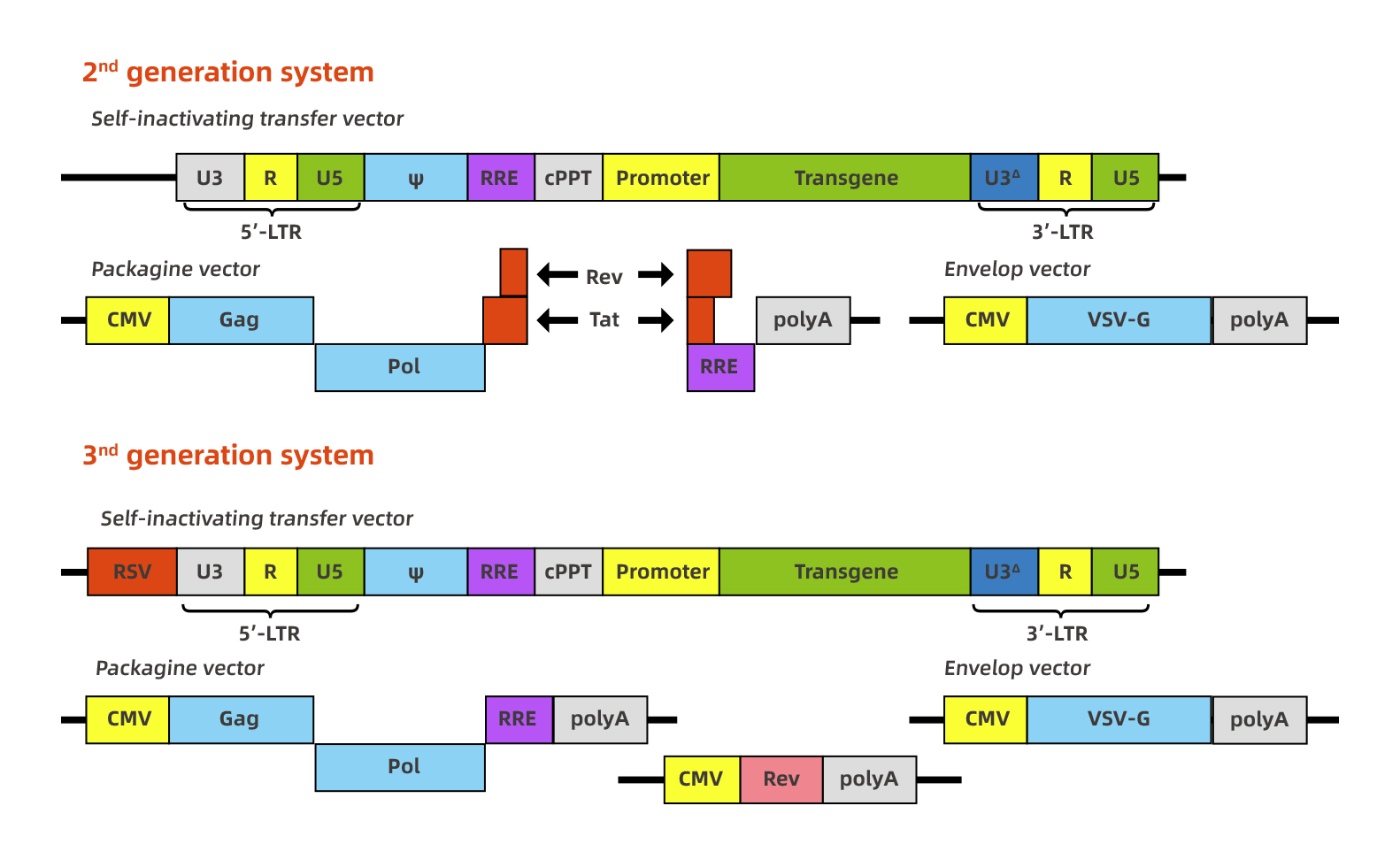
|
|
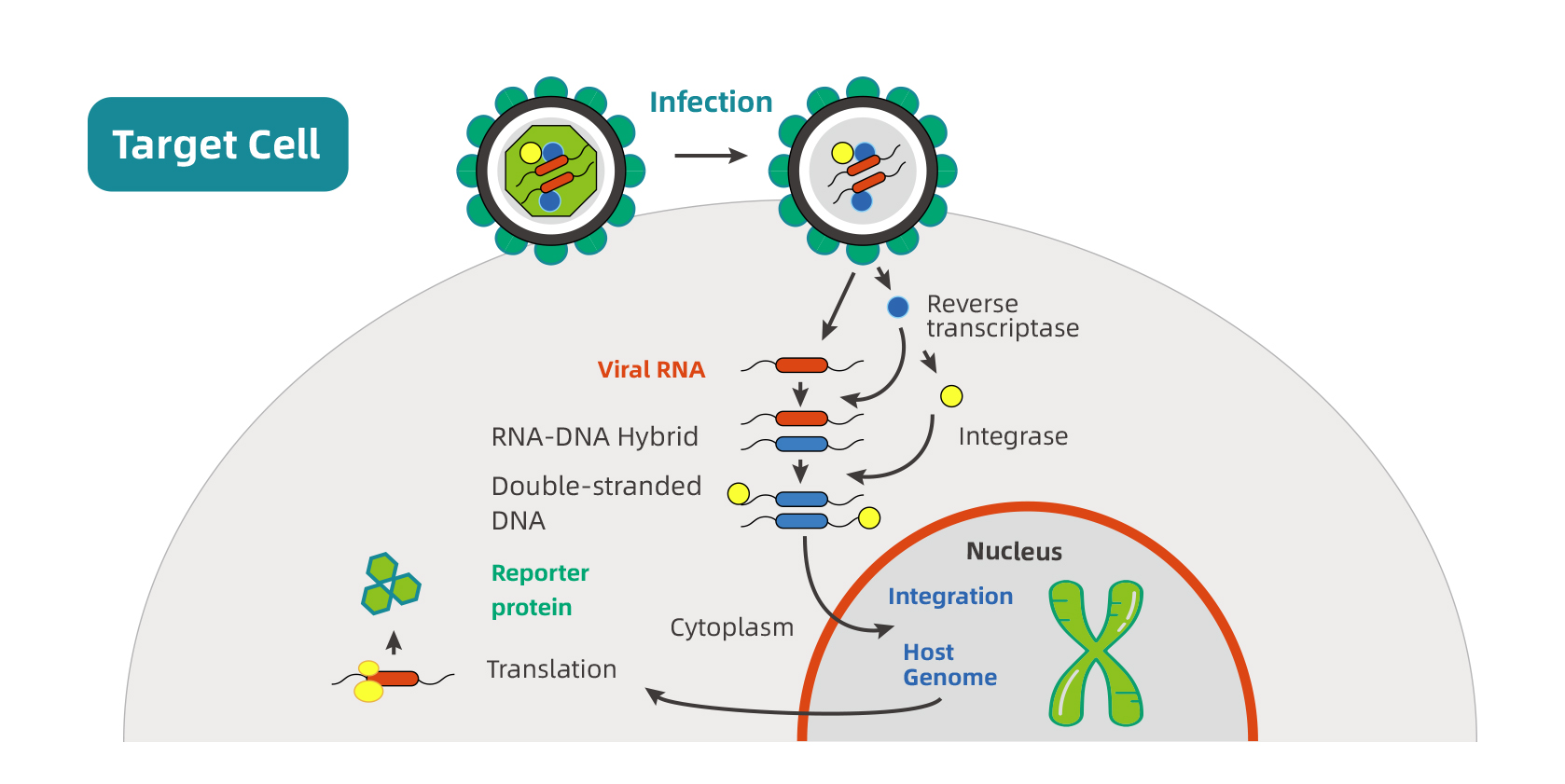
Process of lentivirus infection
The first step in lentiviral infection is the interaction between the viral envelop and the host cell surface receptors. After the viral and cellular membranes fusion, structural, enzymatic proteins, and viral core are released into cytoplasm. The reverse transcriptase (RT) catalyzes the transcription of viral RNA into double-stranded DNA that then forms
a pre-integration complex with the integrase. The pre-integration complex is imported into the nucleus and the viral DNA is integrated into the host genome under the action of integrase. The promoter upstream of the transgene drives its expression in cytoplasm. The schematic below represents the process of lentivirus infection host cells.
|
Characterization of lentivirus:
1. Efficiently transducing a wide range of cells, including dividing and non-dividing cells, especially for some difficult-to-transfect cells (i.e. primary cells, stem cells, undifferentiated cells) and cells resistant to adenoviral infection, such as dendritic cells(DCs), monocytes and mesenchymal stem cells.
2. Integration into host cell genome, mediating stable long-term transgene expression, generating stable cell lines.
3. Wild applications, experiments both in vivo and in vitro, especially for tumor formation experiment in animals.
|
1. Complete vectors:
|
Multiple destination lentiviral vectors are available with different N-terminal or C-terminal fluorescent and affinity tags.
|
|
|
|
2. Quick delivery:
|
Human ORF cDNA clone and Human mirRNA can be shuttled into lentiviral vectors in 2-3 days.
|
|
|
|
3. Excellent seirvce:
|
Based on your research goal, our professional technical staffs will design experimental program and manufacture the most ideal lentivirus particles for you.
|
|
|
|
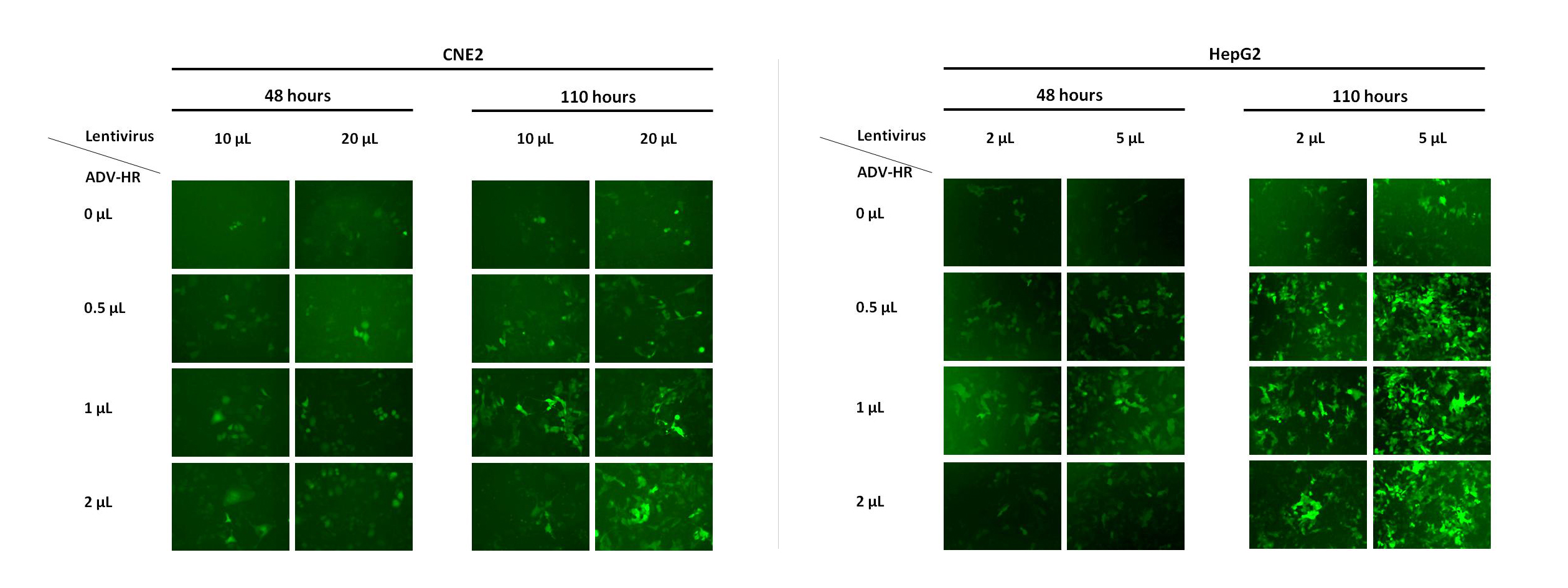
4. Gift:
|
Working with WZ, we will send a free Lentivirus ADV-HR Infection Reagent to you, which can increase infection efficiency for mammalian cells, especially difficult-to-infect cells. The figure below shows the effect of the Lentivirus ADV-HR Infection Reagent in HepG2 and CNE2 cells.
|

|
《Lentivirus technical manual》
|
|
|
1. When to choose lentivirus?
Since lentivirus has a broad host-range, it can infect both dividing cells and non-dividing cells. Lentiviruses can be used as viral vectors in in vivo and in vitro studies. In addition, lentivirus is also an ideal virus for constructing stable transgenic strains.
2. What is the size limit for the ORF that is to be cloned into the lentivirus vector?
In general, lentiviral vectors can accommodate 8 kb inserts. However, when the inserted ORF gene is larger than 4 kb, the packaging efficiency of the virus will be greatly reduced, which will affect the virus titer and even gene expression.
3. What is the amount of cell inoculation used for lentivirus infection?
Inoculate target cells in good condition into a 24-well plate. The cell concentration is generally 1×105/mL cells. Many factors are need to consider when calculate the number of cells to be inoculated, including the cell viability, status, growth speed, division factors, etc., generally to ensure virus infection It is better for the cell confluence rate to reach 50%-70%, when to ensure virus infection.
4. What is MOI?
MOI (multiplicity of infection), the multiplicity of infection, is the ratio of the number of virus particles to the number of cells at the time of infection. It is generally believed that MOI is a ratio without a unit.
5. Can lentivirus express genes stably?
Yes. Lentiviruses can integrate foreign genes into the host genome and will not be lost during cell division and passage. Therefore, the foreign genes can be stable integration and long-term expression.
6. How long is the lentivirus packaging cycle in WZ?
If custom can provide the constructed lentivirus vector, the packaging cycle is around 2 weeks, 5 weeks are need including preliminary vector construction and packaging.
7. After the lentivirus infects the cell, when does the peak expression of the target gene can be observed?
After infecting the target cells with lentivirus, the expression of the target gene can reach to the peak at 72-96h generally, but for some special cells, such as cells with slower proliferation and passage, it takes longer f to reach the peak expression.
8. How to improve the lentivirus infection efficiency?
Good cell growth and a suitable MOI value during infection are the guarantee for high infection efficiency. Generally, the infection efficiency of the virus can be improved by increasing the MOI value during infection. If necessary, the adjuvant agent ADV-HR can be added during infection to improve the infection efficiency.
9. After lentivirus infection, why the cell condition is very poor, and even death?
Lentivirus can cause certain toxicity to cells, and different cells have different tolerance to toxicity. It is recommended to reduce the MOI, to increase the confluence rate of plated cells (up to 70%) during cell preparation and adjust the amount of virus added during infection. In addition, you can also change the medium 4 hours after the infection, and continue the culture and observation with fresh complete culture medium.
|
1.
|
Cell Death & Disease. (IF=6.304). Liu, et al. (2020). Long noncoding RNA SNHG12 promotes tumour progression and sunitinib resistance by upregulating CDCA3 in renal cell carcinoma.
|
|
|
2.
|
Cell Death & Disease. (IF=6.304). Liu, et al. (2020). Activation of STAT3 is a key event in TLR4 signaling-mediated melanoma progression.
|
|
|
3.
|
Cell Death & Disease. (IF=6.304). Wang, et al. (2019). LXRα promotes cell metastasis by regulating the NLRP3 inflammasome in renal cell carcinoma.
|
|
|
4.
|
Biochemical and Biophysical Research Communications. (IF=2.985). Zhu, et al. (2019). MicroRNA-506 inhibits the proliferation and invasion of mantle cell lymphoma cells by targeting B7H3.
|
|
|
5.
|
Molecular Cancer. (IF=15.302). Tan, et al. (2019). PIWI-interacting RNA-36712 restrains breast cancer progression and chemoresistance by interaction with SEPW1 pseudogene SEPW1P RNA.
|
|
|
6.
|
Advanced Science. (IF=15.84). Lai, et al. (2018). A Modular Assembly of Spinal Cord–Like Tissue Allows T argeted Tissue Repair in the T ransected Spinal Cord.
|
|
|
7.
|
Theranostics. (IF=8.579). Mai, et al. (2018). PIWI-interacting RNA-54265 is oncogenic and a potential therapeutic target in colorectal adenocarcinoma.
|
|
|
8.
|
Toxicology Letters. (IF= 3.569). Yan, et al. (2018). Inhibitory Effect of PXR on Ammonia-induced Hepatocyte Autophagy via P53.
|
|
|
1.
|
Cell Death & Disease. (IF=6.304). You, et al. (2020). GADD45α drives brown adipose tissue formation through upregulating PPARγ in mice.
|
|
|
2.
|
Journal of Neuroinflammation. (IF=5.793). Zeng, et al. (2019). Lentivirus-mediated downregulation of α-synuclein reduces neuroinflammation and promotes functional recovery in rats with spinal cord injury.
|
|
|
3.
|
Cell Discovery. (IF=6.255). Liao, et al. (2019). USP10 modulates the SKP2/Bcr-Abl axis via stabilizing SKP2 in chronic myeloid leukemia.
|
|
|
4.
|
International Journal of Biological Sciences. (IF=4.858). Zheng, et al. (2019). Cathelicidin-related antimicrobial peptide protects against cardiac fibrosis in diabetic mice heart by regulating endothelial-mesenchymal transition.
|
|
|
5.
|
Oncogene. (IF=7.971). Liao, et al. (2018). Growth arrest and apoptosis induction in androgen receptor-positive human breast cancer cells by inhibition of USP14-mediated androgen receptor deubiquitination.
|
|
|
6.
|
J Mol Cell Cardiol. (IF= 4.133). Li, et al. (2017). TCONS_00075467 modulates atrial electrical remodeling by sponging miR-328 to regulate CACNA1C.
|
|