mRNA in vitro transcription synthesis service
Utilize professional advantages to expand service areas! Weizhen Biotechnology lay out the mRNA track. With mature and stable processes, they have developed and established a comprehensive mRNA process platform to ensure efficient in vitro transcription synthesis of mRNA. We can provide customers with a complete set of services from gene synthesis, efficient in vitro transcription template preparation, plasmid linearization, IVT mRNA synthesis, purification and quality inspection to LNP packaging.
Based on the needs of customers at different stages, personalized synthesis preparation is completed. The synthesized mRNA can be used for cell transfection, microinjection, in vitro translation, and RNA vaccine development.
1. Prefabricated and customized IVT mRNA products
The synthesis of IVT mRNA is a core step in the entire mRNA preparation process, and the plasmid templates, transcription and modification processes, and quality control used for in vitro transcription are crucial. Weizhen Biotechnology has a plasmid library platform covering tens of thousands of human genes, which can serve as a spot template for in vitro transcription, saving time in template preparation. In addition, the template plasmid optimization technology that surpasses peers, mature mRNA modification processes, extremely high transcription efficiency, combined with multi-step purification processes during the reaction process, ensure the quality and yield of the final mRNA.
At the same time, in order to accelerate the development process of mRNA, a variety of mRNA pre products are provided, covering:
① MRNA expression of high-sensitivity NeoGreen+teLuciferase dual light system reporter gene;
② MRNA expression of GFP and RFP reporter genes;
③ MRNA expressing highly sensitive calcium ion receptors;
④ Expressing mRNA of Cas9/12/13/14;
⑤ MRNA expressing COVID-19 spike protein (full length).
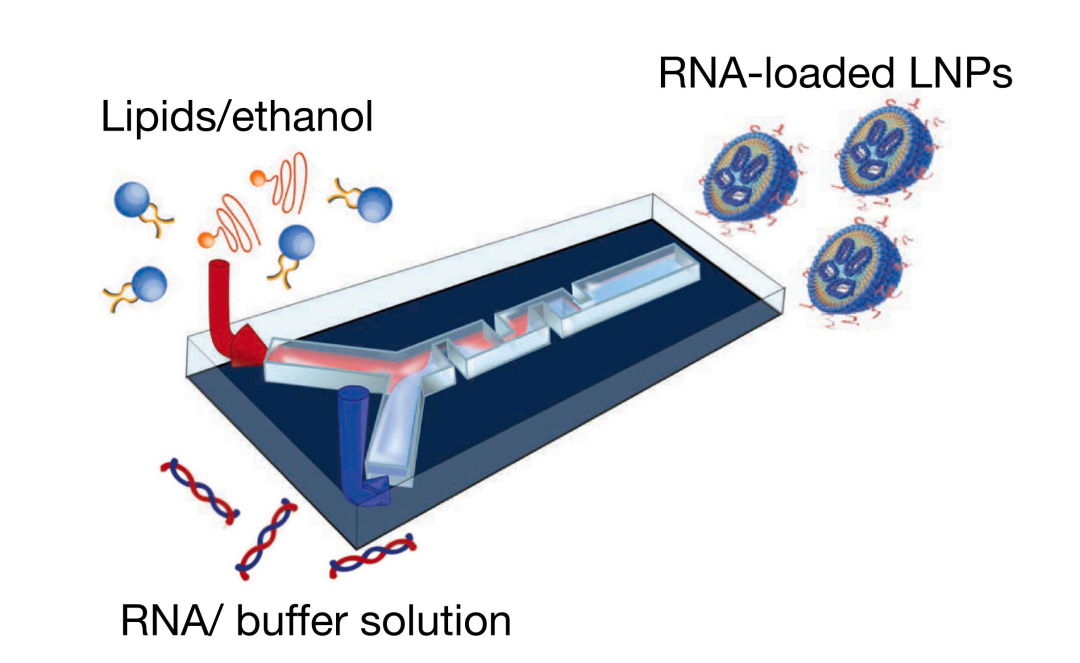
2. Efficient preparation of mRNA LNP using microfluidic technology
In addition to the synthesis and modification of mRNA, how to safely and effectively deliver mRNA from extracellular to intracellular is also a key and difficult point in the development of mRNA technology. We use a simple, fast, mild conditioned, and easily producible microfluidic technique for the preparation of mRNA liposomes (mRNA LNP), encapsulating mRNA in lipid nanoparticles LNP to effectively protect mRNA and transport it to cells for functional purposes.
The main process steps of mRNA preparation include mRNA encapsulation/loading, complex purification, sterilization filtration, and sterile filling. In these processes, the control of aseptic contamination is crucial, taking into account various aspects such as equipment, personnel, materials, environment, and operating methods. Operations such as aseptic filling, plugging, and transfer when the product is not completely sealed all need to be carried out in an A-level environment. The Yiming Cell fully automatic preparation filling line can achieve multi batch filling, and the filling environment is an absolute A-level environment with a fully enclosed isolator.
The mRNA LNP we produce has the following characteristics:
① Good batch consistency;
② Controllable particle size (within 50-200nm);
③ The encapsulation effect can reach over 80%;
④ We can provide mRNA products ranging from mg to g levels for our customers.
Preparation of RNA-LNP using microfluidic technology
(Microfluidic technologies and devices for lipid nanoparticle-based RNA delivery, 2022)
3. Rich quality testing to meet customer needs at all stages
In theory, we can provide customers with the preparation of mRNA and mRNA LNP for any protein. In addition, we can also provide customers with multi-dimensional mRNA detection services, including but not limited to:
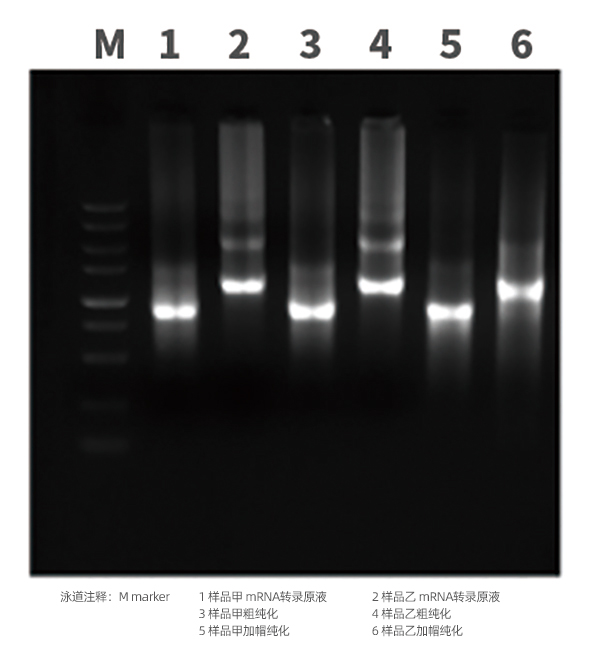
1)mRNA integrity and purity testing
mRNA Identification
mRNA/LNP Quality Control (HPLC)
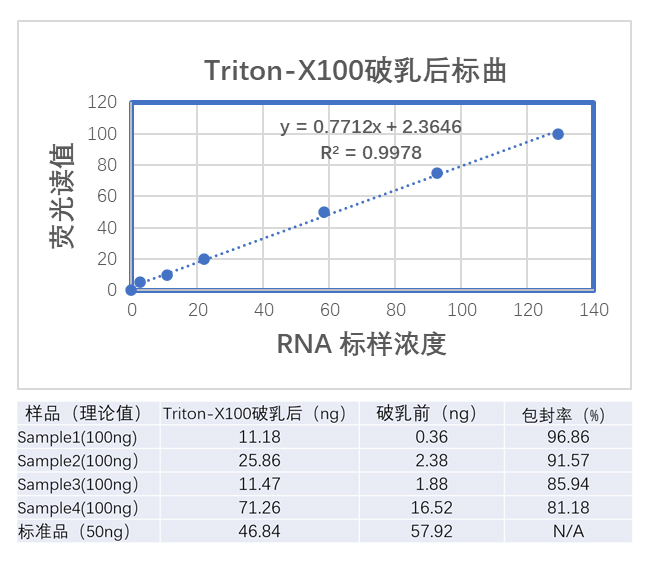
2)LNP packaging rate detection
Packaging Rate Detection
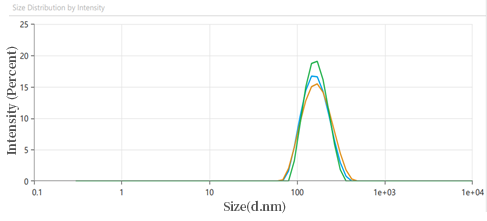
3)LNP particle size detection
mRNA-LNP particle size analysis
(Z-Average(nm):153.2;Polydispensity index (PI): 0.1502)
4) More customized testing (optional)
a) Residual detection (such as T7 RNApol, DNaseI, RNase inhibitor, dsRNA/DNA/linear RNA, etc.)
b) mRNA cap structure detection
c) Cytological expression test
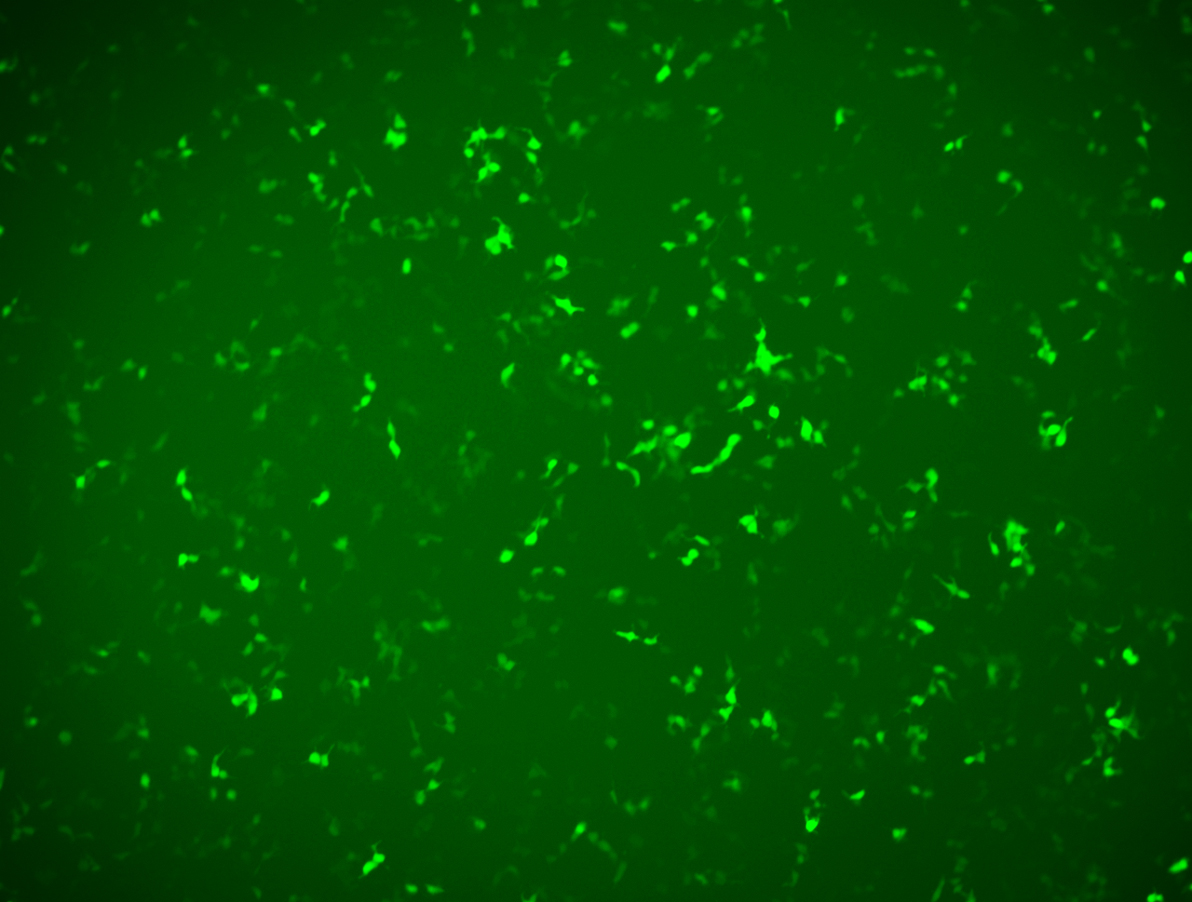
d) Animal level expression test
Validation of mRNA GFP expression effect
4. IVT mRNA Service Process
01 Efficient in vitro transcription template construction (customer supplied plasmids: 2 weeks ; direct synthesis: 4 weeks)
02 In vitro transcription capping, modification purification(2 weeks)
03 Liposome packaging(1 week)
04 Quality inspection report (Cell level: 1 week Animal level: 3 weeks)
5. Service advantages
①. Provide prefabricated or customized mRNA to meet the diverse needs of customers;
②. Microfluidic technology is used for the preparation of mRNA LNP, with high packaging efficiency and easy production scaling up;
③. Possess a transcription template plasmid library, providing one-stop production from gene synthesis to mRNA synthesis;
④. Professional sequence optimization and modification techniques significantly reduce immunogenicity and improve expression efficiency;
⑤. Provide diversified QC processes for project requirements, which can be used for scientific research experiments and assist clinical research;
⑥. Possess GMP plasmid DNA raw material production platform and mRNA raw material production platform;
⑦. The fully enclosed isolator automated filling line can meet the filling requirements of different formulations;
⑧. mRNA one-stop CRO and CDMO overall solution, full process quality management.