Hyperthyroidism modeling tool - Ad-TSHR289
Hyperthyroidism is a common organ specific autoimmune disease caused by excessive synthesis and release of thyroid hormones by the thyroid gland, leading to metabolic hyperactivity and sympathetic nervous system excitation in the body. There are many causes of hyperthyroidism, which are related to emotions, iodine nutrition, autoimmunity, and familial inheritance. Common ones include diffuse toxic goiter (also known as Graves' disease, GD), inflammatory hyperthyroidism, drug-induced hyperthyroidism, HCG related hyperthyroidism, and pituitary TSH thyroid adenoma hyperthyroidism. Among them, Graves' hyperthyroidism is the most common, accounting for about 60% to 80% of all hyperthyroidism.
Graves hyperthyroidism has an annual incidence rate of about 4/10000. The key to its incidence is that under the interaction of environmental and genetic factors, the body's immune system produces autoantibodies (TSAb) against thyroid stimulating hormone receptor (TSHR). The combination of TSAb and TSHR activates the downstream signal cascade reaction, mimicking the continuous stimulation effect of TSH, leading to hyperthyroidism. If not treated in time, it will lead to high incidence rate and mortality. In addition, Graves' hyperthyroidism related eye diseases afflict approximately 50% of patients and are particularly difficult to treat.
1. Thyroid stimulating hormone receptor (TSHR)
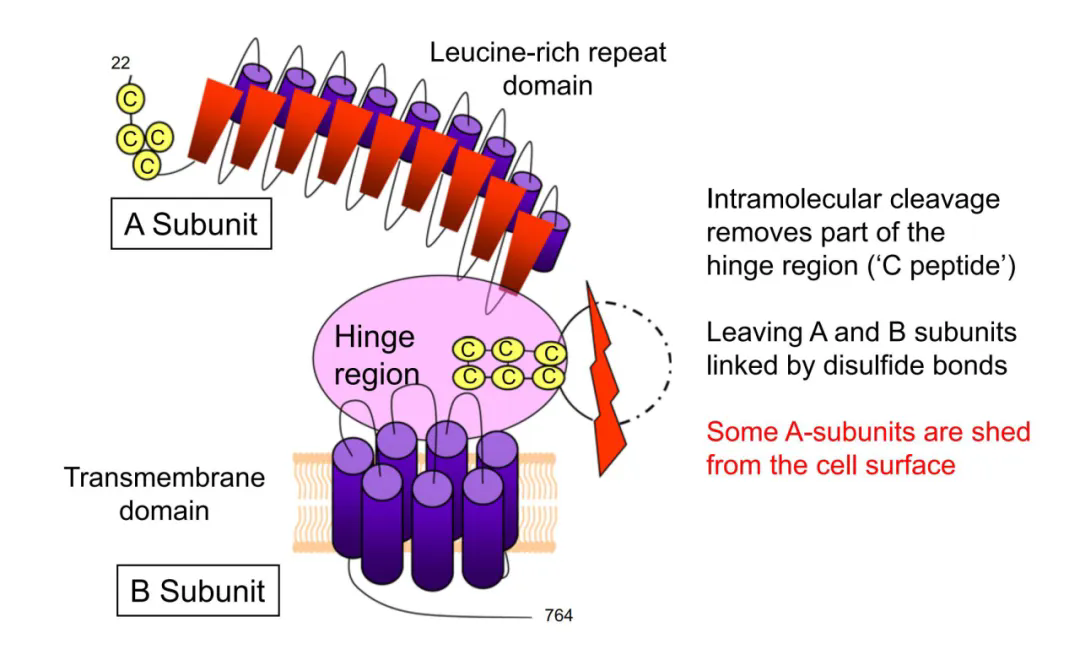
TSHR is a cell surface glycoprotein receptor belonging to the G protein coupled receptor (GPCR) family, consisting of 764 amino acids and a molecular weight of 87 kDa. The human TSHR coding gene is located on the long arm of chromosome 14 (14q31), with a total of 10 exons. The first nine exons encode a large N-terminal region outside the cell, which contains a leucine rich repetitive domain connected to the transmembrane domain through a hinge region; The tenth exon encodes seven transmembrane fragments and a short C-terminus within the cell. Unlike other glycoprotein hormone receptors, TSHR contains a sequence of 50 amino acids (C-peptide) inserted into its extracellular domain. During post-translational modification, the intramolecular cleavage mediated by metalloproteinases separates the N-terminal extracellular domain of TSHR from the transmembrane helical domain, and the C-peptide sequence is removed, converting TSHR into the A subunit (289aa) and B subunit connected by disulfide bonds.
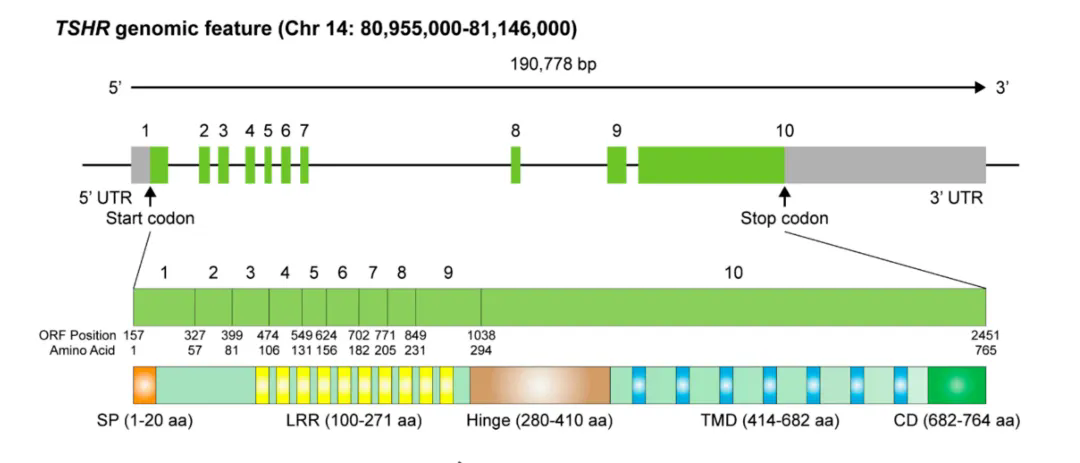
Schematic diagram of TSHR genome structure
Schematic diagram of TSHR protein structure
2. TSHR autoantibodies (TRAb)
TRAb is a thyroid autoantibody that specifically targets TSHR on the surface of thyroid cell membrane. According to different physiological functions, TRAb can be divided into thyroid stimulating antibodies (TSAb) and thyroid stimulating blocking antibodies (TBAb). TSAb is present in the serum of patients with Graves' disease, and when combined with TSHR, it produces a biological effect similar to TSH, stimulating the development of hyperthyroidism in the thyroid gland. It is currently the preferred screening method for GD; TBAb exists in patients with autoimmune thyroiditis and hypothyroidism, and can bind with TSHR to block the action of TSH, leading to hypothyroidism.
3. The pathogenesis of GD
TSHR is mainly expressed in thyroid cells, and its natural ligand is TSH. Under physiological conditions, the extracellular domain of TSHR inhibits the activity of the transmembrane helical domain. When the extracellular domain is specifically bound by TSH, its conformation changes and transforms into an agonist of the transmembrane helical domain, leading to TSHR activation and downstream intracellular signaling cascade reactions to control thyroid growth and synthesis and secretion of thyroid hormones. Under pathological conditions, the structure of TSHR undergoes abnormal changes. Under enzymatic hydrolysis, the disulfide bond connecting subunits A and B is dissolved and broken, and TSHR-A subunit is shed and expressed on the cell surface, inducing and enhancing autoimmune reactions. TSHR acts as a self antigen to stimulate the body to produce antibody TSAb, leading to the occurrence of hyperthyroidism. Therefore, the immunogenic TSHR-A subunit is the key to inducing autoimmune responses, and research on the TSHR-A subunit has become a hot topic in GD model studies.
4. GD animal modeling method
At present, the pathogenesis of GD is not clear. Establishing an animal model that conforms to the characteristics of GD is of great significance for the diagnosis and treatment of the disease. Exogenous thyroxine supplementation and immune induced goiter are the main methods for establishing induced hyperthyroidism animal models, among which the recombinant adenovirus expressing human TSHR-A subunit (Ad-TSHR-289) immune induced hyperthyroidism model is an ideal and widely used GD animal model.
Reference Cases
The thyrotropin receptor autoantigen in Graves disease is the culprit as well as the victim(The Journal of Clinical Investigation,IF 15.9)
Experimental methods
① Construction and in vivo injection of adenovirus expression vector
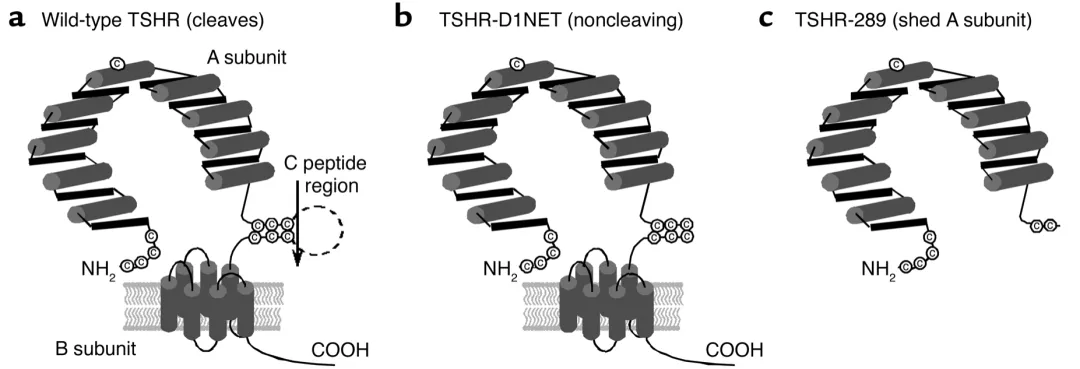
Researchers constructed adenovirus encoding TSHR free A subunit (TSHR-289) and TSHR full-length (TSHR-D1NET, with minimal subunit cleavage), and used Ad - β - gal and Ad TSHR-WT as controls. Inject the above-mentioned adenovirus into 6-7 week old female BALB/c mice via muscle injection, with a virus dosage of 1011 particles and an injection volume of 50 μ L PBS suspension.
Three different structural forms of TSHR loaded with adenovirus
② Adenovirus immunization method
Adenovirus was injected into mice three times, with a three week interval between each injection. Blood was drawn one week after the second injection, and the animals were euthanized 8 weeks (first experiment) or 4 weeks (second experiment) after the third injection to obtain blood and thyroid gland.
experimental result
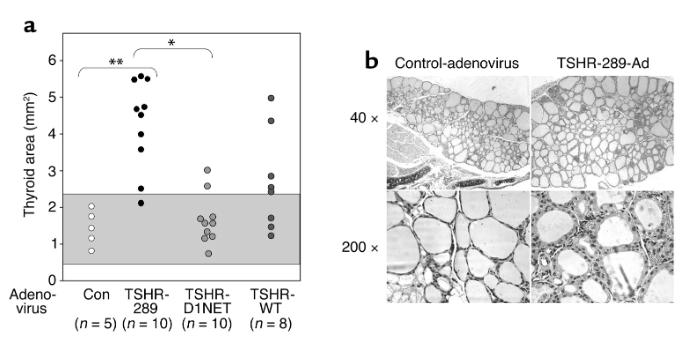
After secondary immunization, serum thyroid hormone levels were detected and found to be elevated in 80% of mice in the Ad-TSHR-289 group, while only 10% in the Ad-TSHR-D1NET group; Similar results were obtained after three immunizations. Histological examination showed that 90% of Ad-TSHR-289 immunized mice exhibited significant thyroid enlargement, while only 20% of Ad-TSHR-D1NET immunized mice showed thyroid enlargement. Mice immunized with Ad TSHR-WT exhibited a response between the two.
Thyroid swelling and histological comparison in mice injected with TSHR adenovirus
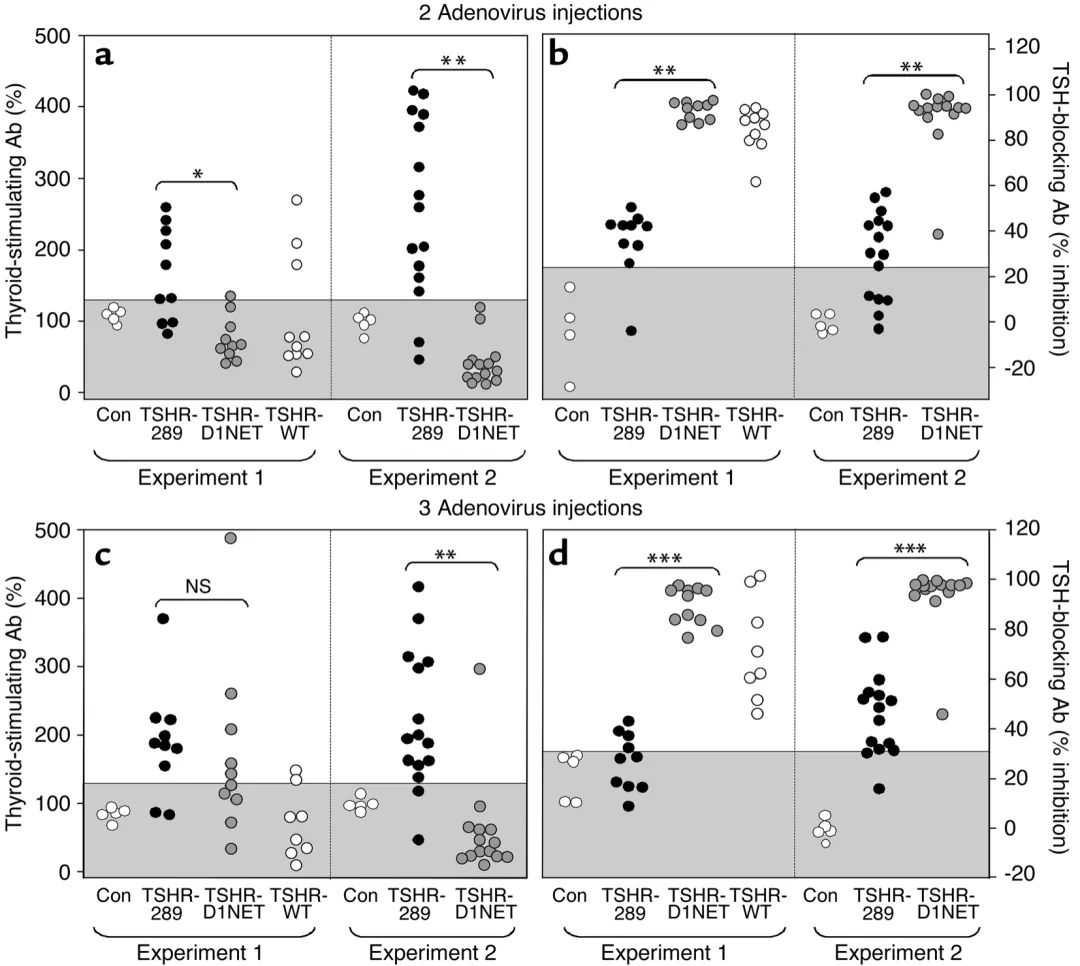
The cAMP response measurement data showed that the TSAb activity in the serum of Ad-TSHR-289 group mice was significantly higher than that of Ad-TSHR-D1NET group after secondary immunization, while the TBAb activity was significantly lower than that of Ad-TSHR-D1NET group. The levels of TSAb and TBAb in the Ad TSHR-WT group were between the two groups.
TSAb and TBAb in adenovirus immunized mice expressing different structural forms of TSHR
The above data indicates that the free TSHR-A subunit has stronger immunogenicity and can more effectively induce the onset of GD in mice compared to the full-length TSHR, with a higher modeling rate.
Weizhen Biological Ad-TSHR in stock·
Human TSHR gene adenovirus in stock, with high titer and short cycle, helps establish immune induced hyperthyroidism animal models and supports hyperthyroidism research!